Abstract
Background
In patients with nontraumatic osteonecrosis of the femoral head (ONFH), implantation of bone marrow aspirate concentrate (BMAC) could delay the progression of osteonecrosis and improve symptoms in pre-fracture ONFH. However, the BMAC content, especially in osteoblastic stem cells, could have an important individual variability. An autologous osteoblastic cell product could improve the effect of such cell-based therapy.
Questions/purposes
(1) Does autologous osteoblastic cell therapy decrease the likelihood of progression to subchondral fracture with or without early collapse corresponding to Association Research Circulation Osseous (ARCO) classification Stage III or higher, and provide a clinically important pain improvement compared with BMAC treatment alone? (2) Were patients treated with osteoblastic cell therapy less likely to undergo subsequent THA? (3) What proportion of patients in the treatment and control groups experienced adverse events after surgery?
Methods
Between 2004 and 2011, we treated 279 patients for Stage I to II hip osteonecrosis (ON) with surgery. During that time, our general indications for surgery in this setting included non-fracture ON lesions. To be eligible for this randomized, single-blind trial, patients needed to have an ONFH Stage I or II; we excluded those with traumatic ONFH, hemoglobinopathies and positive serology for hepatitis B, C or HIV. Of those treated surgically for this diagnosis during the study period, 24% (67) agreed to participate in this randomized trial. Hips with pre-fracture ONFH were randomly treated with a core decompression procedure associated with either implantation of a BMAC (BMAC group; n = 26) or osteoblastic cell (osteoblastic cell group; n = 30). The groups were not different in terms of clinical and imaging characteristics. The primary study outcome was treatment response, defined as the absence of progression to subchondral fracture stage (ARCO stage III or higher) plus a clinically important pain improvement defined as 1 cm on a 10-cm VAS. The secondary endpoint of interest was the frequency in each group of subsequent THA and the frequency of adverse events. The follow-up duration was 36 months. We used an as-treated analysis (rather than intention-to-treat) for our efficacy endpoint, and an intention-to-treat analysis for adverse events. Overall, 26 of 26 patients in the BMAC group and 27 of 30 in the osteoblastic cell group completed the trial.
Results
At 36 months, no clinically important differences were found in any study endpoint. There was no difference in the proportion of patients who had progressed to fracture (ARCO stage III or higher; 46% of the BMAC hips [12 of 26] versus 22% in the hips with osteoblastic cells [six of 27], hazard ratio, 0.47 [95% CI 0.17 to 1.31]; p = 0.15). There was no clinically important difference in VAS pain scores. No differences were found for either the WOMAC or the Lequesne indexes. With the numbers available, there was no difference in the proportion of patients in the groups who underwent THA at 36 months 15% (four of 27) with osteoblastic cells versus 35% (nine of 26) with BMAC; p = 0.09 With the numbers available, we found no differences between the treatment and control groups in terms of the frequencies of major adverse events.
Conclusions
We found no benefit to osteoblastic cells over BMAC in patients with pre-collapse ONFH; side effects were uncommon and generally mild in both groups. This study could be used as pilot data to help determine sample sizes for larger (presumably multicenter) randomized controlled trials. However, this novel treatment cannot be recommended in routine practice until future, larger studies demonstrate efficacy.
Level of Evidence
Level II, therapeutic study.
Introduction
Nontraumatic osteonecrosis of the femoral head (ONFH) is characterized by epiphyseal necrosis of the osteomedullary tissue. This can cause pain, subchondral fracture, femoral head collapse, and lead to THA. Because many patients with nontraumatic ONFH are young, surgeons generally seek to avoid THA when possible in these patients [17]. Bone cell and/or mesenchymal cell (MSC) deficiency may exist in patients with ONFH [5, 9]. An inability to repair lesions in the affected areas results in local structural weakness because of weightbearing stress and eventually bone collapse [19].
Autologous bone marrow cell therapies tested in the early stages ONFH, mainly autologous bone marrow aspirate concentrate (BMAC), have had encouraging results [3, 4, 10, 12]. BMAC contains MSCs and endothelial progenitor cells that have a number of potentially beneficial biological properties, and some research suggests efficacy of treatments with these cells may be related to the number of MSCs implanted [12]. However, the non-cell part of BMAC, including cytokines and growth factors, may also play a role. Furthermore, the number of MSCs in the BMAC depends on several factors, such as age, and ON etiological factors, including alcohol abuse and corticotherapy. Another approach could be the use of a preliminary ex-vivo processing of the autologous bone marrow to increase the number of MSCs and to boost the osteoblastic differentiation. We attempted to investigate this by comparing, in a randomized setting, BMAC with a pure cell product including osteoblasts.
Specifically, we asked: (1) Does autologous osteoblastic cell therapy decrease the likelihood of progression to subchondral fracture with or without early collapse corresponding to Association Research Circulation Osseous (ARCO) Stage III or higher and provide a clinically important pain improvement compared with BMAC treatment alone? (2) Were patients treated with osteoblastic cell therapy less likely to undergo subsequent THA? (3) What proportion of patients in the treatment and control groups experienced adverse events after surgery?
Patients and Methods
Study Design and Patients
This randomized, single-blind (observers), exploratory study was conducted in two centers in Belgium. We included patients aged 18 years or older and those with ONFH of pre-fracture Stage I or II according to ARCO [18], confirmed at screening by radiographs and MRI. We also enrolled patients with bilateral hip ONFH. We excluded patients with traumatic ONFH or hemoglobinopathies and those with positive serology results for hepatitis B, C, or HIV. The study was approved by the ethics committees of the two institutions (ClinicalTrials.gov identifier: NCT02890537); all patients provided written informed consent.
Between 2004 and 2011 we treated 279 patients for Stage I to II hip osteonecrosis (ON) with surgery. During that time, our general indications for surgery in this setting included non-fracture ON lesions. Of those treated surgically for this diagnosis during the study period, 24% (67) agreed to participate in this randomized trial (Fig. 1).
Fig. 1.
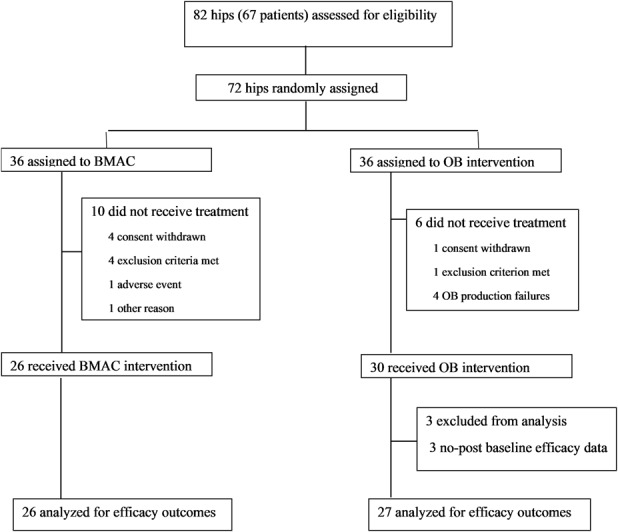
This figure shows the CONSORT flow diagram of the trial; BMAC = bone marrow aspirate concentrate; OB = osteoblast.
Randomization and Masking
Hips were randomly assigned in a 1:1 ratio to either BMAC (the BMAC group; n = 36) or osteoblastic cell implantation (the osteoblastic cell group; n = 36). A single randomization list was generated for the two sites. When both hips of the same patient were enrolled in the study, the right hip was assigned to the treatment arm from the first available envelope. The left hip was then assigned to the other treatment arm. Patients and treating physicians were not blinded to the treatment allocation because the procedure schedules were different. Clinical research assistants and radiologists were masked to the treatment assignment. We used an as-treated analysis (rather than intention-to-treat) for our efficacy endpoint, and an intention-to-treat analysis for adverse events.
Populations
Sixty-seven patients with 82 hips affected by ONFH were enrolled in the study (Fig. 1). Randomization was done for 72 hips (59 patients). Assigned treatment was not done in 16 hips (16 patients), due to ineligible subchondral fracture stage ONFH (n = 5), withdrawal of patient’s consent (n = 5), production failure of the osteoblastic cells (n = 4), acute viral hepatitis (n = 1), and a protocol violation (n = 1). Fifty-six randomized hips underwent either BMAC implantation (26 hips) or osteoblastic cell implantation (30 hips). Eight patients were treated in the study concomitantly on both hips, with osteoblastic cells on one hip and BMAC on the other hip. For the efficacy set, we excluded three patients from the osteoblastic cell group due to the absence of follow-up after treatment. Thus, we analyzed efficacy data for 53 hips, comprising 27 hips in the osteoblastic cell group and 26 hips in the BMAC group. For the secondary outcomes, we included a total of 63 treated hips, comprising 33 hips in the osteoblastic cell group and 30 hips in the BMAC group.
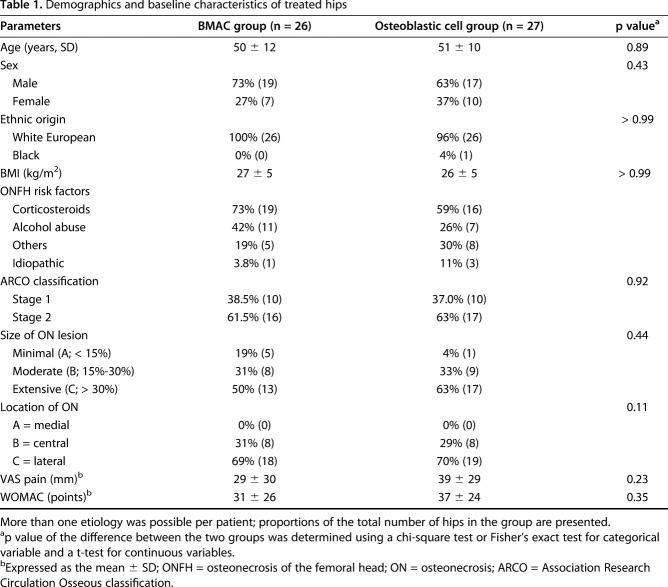
At baseline, clinical and imaging characteristics were not different between the groups (Table 1).
Table 1.
Demographics and baseline characteristics of treated hips
Procedures
In the BMAC group, BMAC was prepared from the patient’s bone marrow on the day of the study treatment. These procedures have been previously published [4]. Under general anesthesia, a volume of 400 ± 85 mL autologous bone marrow was harvested from the anterior or the posterior iliac crests; bone marrow was then sorted on a Spectra cell separator (777006300; Cobe, Lakewood, CO, USA) and concentrated to a final volume of 40 ± 11 mL. This amount of bone marrow was recommended to obtain a maximum of MSCs in the BMAC for boosting bone reconstruction [12]. During the same general anesthesia, a special 3-mm trephine was inserted in the necrotic lesion under control of a fluoroscopic view through the great trochanter, the neck, and the femoral head. Then the BMAC was injected into the necrotic region. To avoid any leakage, a piece of Gelfoam® absorbable gelatin sponge (Pharmacia & Upjohn Company, Kalamazoo, MI, USA) was pushed through the trephine to close the hole. An aliquot of the BMAC was systematically analyzed for determining the number of mononuclear cells, the number of MSC using fibroblast colony-forming units (CFUs) method, and the number of CD34-positive cells (precursor of hematopoietic stem cells). In this study, BMAC contained 3.4 ± 3.0 x 109 nucleated cells, including 0.6 ± 0.2 % CD34+ cells and 9.2 ± 9.5 x 106 CFU-F. A culture was systematically done to control the sterility.
In the osteoblastic cell group, the osteoblastic cells were manufactured by the Unité de Thérapie Cellulaire et Moléculaire and the Laboratoire de Thérapie Cellulaire et Génique for products manufactured from and to be administered to patients enrolled at the two facilities in this study. Under local anaesthesia, 49 ± 18 mL of bone marrow were harvested from the posterior iliac crest. During a 3-week procedure, MSCs were isolated, expanded, and differentiated ex vivo under autologous conditions to obtain a population of osteoblastic cells (European Patent 001360, 2006-02-1). After 3 weeks, patients underwent a core decompression as described above, and a target dose of 20.106 osteoblastic cells diluted in 5 mL of saline solution was implanted in the necrotic lesion through the same trephine used for the core decompression. In a biodistribution sub-study, osteoblastic cell pharmacokinetics was studied in four hips (from four patients), using radio-labelled [111Indium] cells injected into the necrotic lesion of the femoral head [7]. At 2, 4, 24 and 48 hours post-implantation, 20-minute static positron emission tomography scans were taken in the area of the iliac crest and in the total body to assess the number of osteoblastic cells retained at the implantation site and to determine their pharmacokinetic profile. Respectively, at 4, 24 and 48 hours post-implantation, radio-labelled osteoblastic cells were detected in femoral bone including the femoral head, proximal femoral metaphysis, and osteomedullary compartment (67%, 61%, 61%), in the lungs (mean 13%, 1%, 0%), and in the liver and spleen (mean 20%, 39%, 39%).
The mesenchymal phenotype of osteoblastic cells was controlled by flow cytometry for positive MSC features (CD105, CD73, CD90) and negative hematopoietic stem cell markers (CD45, CD19, CD14) [2]. The osteoblastic character was also controlled: the CD166 expression using flow cytometry was 60 ± 0%, the alkaline phosphatase enzymatic activity was 1 ± 1 mU/mg/protein, and the secretion of type 1 procollagen (N-terminal) (P1NP) was 48 ± 32 ng/mL as measured by ELISA. The mineralization capacity of osteoblastic cells was demonstrated by a strong Alizarin red staining, corresponding to more than 60% of the 10 cm2 well [1]. Release of osteoblastic cells was done according to the following criteria: cell viability (> 80%), quantity of viable cells (manual counting) and a sterility test (gram staining). Of the 33 hips treated with osteoblastic cells, 27 were implanted with 20 million cells (planned dose) and six were implanted with a lower cell dose (range 1.5-18 million cells). All osteoblastic cell products conformed to specifications, including sterility. For 11 osteoblastic cell products, karyotyping was assessed at two timepoints; first, when cells were dividing during the manufacturing process, and second, at the end of the manufacturing process. When a sufficient number of cells was observed, culture was stopped, and cells fixed and prepared for Q-banded karyotype analysis. Ideally, 15 metaphases per sample had to be analyzed for structural and numerical chromosomal pattern (Cytovision software, Leika Biosystems, Diegem, Belgium). A total of 12 karyotype analyses of nine different osteoblastic cell products were found conclusive. All batches had a normal karyotype (no clonal abnormality in the sample), except two: one karyotype displayed a trisomy of chromosomes 2 and 8 in two metaphases; one karyotype displayed a trisomy of chromosome 2 in 3 metaphases based on the analysis of 14 metaphases. These two patients had a medical history of tumor in the past 5 years at study inclusion (breast cancer and meningioma). Therefore, karyotypes prove that potential chromosomal abnormalities in osteoblastic cells are uncommon and random, with no evidence of specific cell transformation induced by the process. In addition, there was no evidence of tumor development or neoplastic changes in the patients during follow-up.
Outcomes
The primary outcomes were the absence of progression to subchondral fracture stages and a clinical improvement. A composite outcome called “treatment responders” was used, including the absence of progression to a subchondral fracture stage combined with clinically relevant hip pain relief. The secondary outcomes were conversion to THA and adverse events.
Patients were assessed at baseline and at 36 months for clinical outcomes using the WOMAC (total score and subscale scores for pain, stiffness, and function), Lequesne index, and a Likert-type VAS score for pain, and radiologic ONFH progression was assessed using radiography (AP and frog-leg views). The size and location of the necrotic lesion in the femoral head were assessed at baseline using MRI. The ARCO stage [16] was determined for each hip at baseline and at each interval of the follow-up period by four blinded readers . We also used a composite outcome following the recommendations of the regulatory agencies (ICH E9, CPMP/EWP/908/99): the treatment responders. A treatment responder did not subsequently have a subchondral fracture and experienced clinically relevant pain relief (minimum 10-mm VAS pain improvement) [13, 15]. Patients with a baseline VAS pain score less than 10 mm needed a score of 4 mm or less to be considered as having responded well clinically, in accordance with the result of a previous study [10]. A decrease in the WOMAC score of at least 2 points was considered a clinically relevant improvement in function.
The decision to turn to THA when the treatment had been insufficient in controlling pain and/or disability was discussed with the patient at the end of each assessment session. The final decision was taken according to the patient’s own wishes. The tolerability of osteoblastic cells and BMAC were monitored throughout the study period.
Follow-up
The mean follow-up was 36 months (range 35-38). At 36 months, 100% (26 of 26) of the treatment group, and 90% (27 of 30) of the control group had complete follow-up, including radiographic imaging and clinical scores both before study initiation and at 36 months. We also checked how many patients had not been seen in the last 5 years (including patients who underwent THA, and patients referred to our centers only for the study): 70% (23 of 33) in the osteoblastic cell group and 73% (22 of 30) in the BMAC group had not been seen in the last 5 years.
Statistical Analysis
Because of the exploratory nature of the study, we planned no formal hypothesis testing and sample size calculation, and we based the sample size on feasibility, considering the rare occurrence of pre-fracture ON. We estimated the difference in proportion between the two groups and exact 95% CI of the difference with a generalized estimating equation regression model. The time to progression to fracture over 36 months was compared between groups using a log-rank Kaplan-Meier analysis. We used a Cox model with treatment effects to estimate the hazard ratio and its 95% CI. Clinical scores (VAS pain, WOMAC, and Lequesne index) were summarized using descriptive statistics; the absolute change in the scores was compared at each studied timepoint using a t-test, with differences (together with the 95% CI) calculated using a linear model for repeated measures. Comparisons were performed with two-sided tests, with p values less than 0.05 considered statistically significant. Because these comparisons were solely intended for exploratory purposes, no adjustment to control the Type I error for multiplicity was performed.
The adverse events set comprised all treated hips. Treatment-emergent adverse events were defined as adverse events that occurred on or after the day of core decompression or drug implantation. Statistical analyses were performed using the SAS software, version 9.3 (Tervuren, Belgium). To investigate whether location and quantification predict ARCO stages, logistic regression analyses were performed with the R software, version 3.4.3 (R foundation, Vienna, Austria).
Results
Primary Outcomes
At 36 months, no clinically important differences were found in any study endpoint. There was no difference in the proportion of patients who had progressed to fracture (ARCO Stage III or higher; 46% of the BMAC hips (12 of 26) versus 22% in the hips with osteoblastic cells (six of 27), hazard ratio 0.47 [95% CI 0.17 to 1.31]; p = 0.15). A Kaplan-Meier analysis over the 3-year period after implantation shows the risk of ONFH progression to fracture was not different between the two treatments (hazard ratio 0.5 [95% CI 0.2 to 1.3]; p = 0.15) (Fig. 2).
Fig. 2.
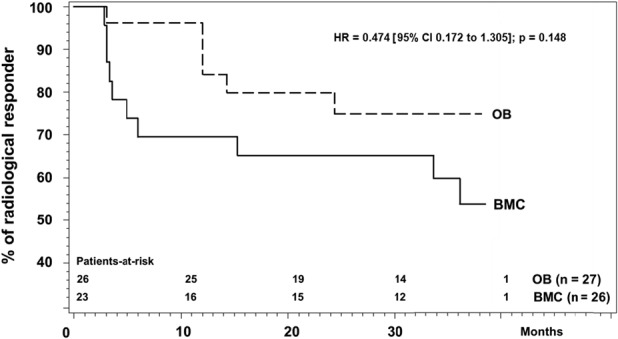
This survival analysis shows the lack of progression to fracture at more than 36 months after treatment.
Likewise, there was no clinically important difference in pain or other outcomes scores between the groups. For improvement in VAS pain, the scores for osteoblastic cells and BMAC were, respectively, 18 mm ± 41 versus 7 mm ± 36 (mean difference 11 [95% CI 34.6 to 1.9]; p = 0.03), but this difference was well below the minimum clinically important difference (MCID) of 20 mm for the VAS scale [14]. For improvement in WOMAC, there were no differences between the osteoblastic cell and BMAC groups (-13 ± 34 versus -4 ± 35, mean difference 9 [95% CI -17.6 to 9.5]; p = 0.11), nor was there a difference in improvement in the Lequesne index (-4 ± 8 versus -2 ± 7%, mean difference 2 [95% CI -3.73 to 2.17]; p = 0.07).
There was no difference in the proportion of patients in the osteoblastic cell and BMAC groups in terms who met the definition of “treatment responder” (fracture plus pain relief). The treatment responder outcome was, at 36 months of follow-up, reached for osteoblastic cell and BMAC implantation, respectively, in 56% (15 of 27) (95% CI 35 to 75) and 39% (10 of 26) (95% CI 20 to 59) (mean difference 0.17 [95% CI -0.1 to 0.4]; p = 0.24).
Secondary Outcomes
The groups did not differ in terms of the proportion of hips that were converted to THA at 36 months (15% [four of 27] for osteoblastic cells and 35% [nine of 26] for BMAC; p = 0.09).
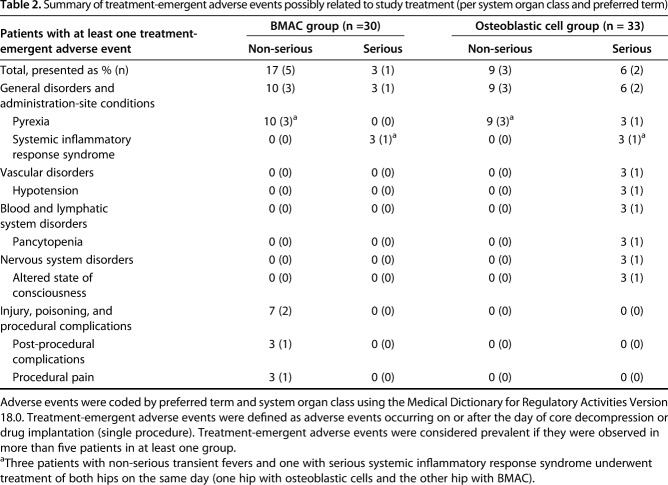
Adverse events generally were uncommon and transient in both groups. With the numbers available, there were no differences between the osteoblastic cell and BMAC groups in terms of the frequencies of major adverse events (Table 2), although we were grossly underpowered on this endpoint. No deaths were reported during the study period. Pyrexia was the most frequent treatment-emergent adverse event (possibly related to the study treatment) reported. Three patients treated bilaterally on the same day experienced transient, mild or moderate episodes of fever, and one patient treated with osteoblastic cells in one hip developed a severe episode of fever, rated as serious, shortly after drug implantation; none were attributable to sepsis.
Table 2.
Summary of treatment-emergent adverse events possibly related to study treatment (per system organ class and preferred term)
Discussion
Clinical investigations assessing the benefits of cell-based therapies for the treatment of ONFH, mostly using BMACs, generally have reported favorable outcomes like pain relief, reduction of evolution to subchondral bone fracture stages, reduction in the proportion of patients converted to THA [3, 10, 12, 20]. Our first trial in non-fracture ONFH compared core decompression alone to core decompression augmented with BMAC [3, 4], and we found BMAC added to core decompression was superior to core decompression alone in terms of pain relief, reduction of evolution to subchondral bone fracture stages, and reduction in the proportion of patients converted to THA. These findings have been reported by others [11]. Although the osteogenic effect of BMAC may be a function of MSCs, it may also be a function of the non-cell part of the BMAC, including cytokines and growth factors [6], although we are not aware of any specific study demonstrating this. Some research suggests that the efficacy of the BMAC treatment is related to the number of implanted MSCs [12], and the number of MSCs can vary widely in BMAC. This points to a limitation of the therapeutic use of autologous BMAC: the inability to deliver sufficient numbers of MSCs to the site of the treated lesion. We therefore developed another approach that produces an adequate number of osteoblastic cells by ex vivo culture, and in this study sought to evaluate its efficacy by comparing BMAC with a pure cell product (osteoblastic cells) in a randomized trial in which both study arms included the same core decompression procedure. However, in this randomized, controlled trial, we found no benefit to the use of osteoblastic cells in terms of the risk of progression to subchondral fracture, pain relief, or conversion to THA.
Limitations
This study had several important limitations. First, it was probably underpowered. We hoped to recruit 100 hips. Over 7 years, 82 hips were assessed for the trial but only 53 hips completed it. In this situation, we encourage the reader to consider both possible interpretations of our no-difference finding: Either there indeed is no difference between the groups, or a difference was present but was not detected because of insufficient sample size. Our data may be used to determine sample size for future trials on this topic. A further limitation is that the follow-up was only for 36 months. We tried to obtain a follow-up at 5 years, but too much data was missing.
Other study aspects may have impacted the results. Concerning the blinding process, the study could not be double blind since treating physicians and patients knew which cell-based therapy was used. Nevertheless, we believe that pain and functional assessments were not affected by this limitation; first, no information on the possible superiority of one cell therapy over the other was provided during the informed consent procedure; and second, the pain and functional assessments were found to be consistent with unbiased blind outcome assessments of radiographic imaging on fracture evolution. However, there remains a risk of more positive reporting bias among patients who received osteoblastic cell.
Patients with bilateral ONFH were included; doing this may violate the assumptions of the statistical tests we used (which assume independence of all observations). However, in non-traumatic ONFH, bilateral ON lesions are frequent [8]. Since each patient with bilateral ONFH served as his or her own control, we believe this is not a severe limitation.
Progression to Fracture and Pain Scores
Concerning the primary outcomes of treatment efficacy (progression to subchondral fracture and improvement in pain scores), we found no benefit of osteoblastic cells over BMAC. In the BMAC group, the results observed were not as good as previously published results [3, 10, 12, 20]. We do not have a robust explanation for this. One possible explanation for the lack of response to osteoblastic cell treatment may have been the delivery of an insufficient number of cells. Future studies might seek to determine whether doses higher than 20.106 osteoblastic cells are more effective than what we observed. Such further tests would require new randomized controlled trial; indeed, no appropriated ON animal models exist to test these treatments.
Secondary Outcomes: THA and Adverse Events
There was no difference between the groups in terms of secondary outcomes, namely the frequency of conversion to THA, and the frequency or severity of adverse events, although we were grossly underpowered on this endpoint. Transient post-implantation fever was reported in both groups. Pyrexia was observed in three patients after bilateral treatment and in one patient treated with OB cells on one hip. Symptoms were resolved within 24 hours without further therapy; this may represent a reaction to cell implantation or to the decompression procedure itself, as reported by others [10, 16]. Karyotypic abnormalities of the kinds we observed in a subset of osteoblastic cell samples in this study remain of unknown clinical importance, but nevertheless deserve further investigation as well as longer prospective follow-up of the treated patients.
Conclusions
Given the fact that osteoblastic cells were no more effective than BMAC for the treatment of ON, and considering the large cost associated with the treatment as well as the question of karyotype abnormalities of unknown importance with osteoblastic cell expansion cultures, we cannot recommend the use of osteoblastic cells for this indication until the importance of karyotypic abnormalities is ascertained and larger studies demonstrate efficacy and safety. Although our study was underpowered, we believe it may serve to inform sample-size calculations for future, larger, multicenter RCTs—perhaps using higher doses of osteoblastic cells—which we think should be conducted because the therapeutic challenge of the early ON stages remains poorly resolved.
Acknowledgments
We thank Eric Thille for producing the cell therapy products used in this study.
Footnotes
This work was supported by the Fonds National pour la Recherche Scientifique and Bone Therapeutics SA (Gosselies, Belgium).
One of the authors certifies that he (J-PH) has a patent for an osteoblast cell product (European Patent 001360, 2006-02-1) and may receive payments or benefits, during the study period, in an amount of less than USD 10,000 from Bone Therapeutics SA.
One author (JI) certifies that he is a former employee of Bone Therapeutics SA, a company that partially financially supported the work.
Each remaining author certifies that neither he or she, nor any member of his or her immediate family, has further funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at Erasme University Hospital, University of Brussels, Brussels, Belgium and the Sart Tilman University Hospital of Liège, University of Liège, Belgium.
References
- 1.Aubin JE, Triffitt JT. Mesenchymal stem cells and csteoblast differentiation. In: Bilezikian JP, Raisz LG, Rodan GA, eds. Principles of Bone Biology . Academic Press; Elsevier, Cambridge, MA, USA: 2002:59-81. [Google Scholar]
- 2.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. [DOI] [PubMed] [Google Scholar]
- 3.Gangji V, De Maertelaer V, Hauzeur JP. Autologous bone marrow cell implantation in the treatment of non-traumatic osteonecrosis of the femoral head: five year follow-up of a prospective controlled study. Bone. 2011;49:1005-1009. [DOI] [PubMed] [Google Scholar]
- 4.Gangji V, Hauzeur JP. Treatment of osteonecrosis of the femoral head with implantation of autologous bone-marrow cells. Surgical technique. J Bone Joint Surg Am. 2004;86:1153-1160. [DOI] [PubMed] [Google Scholar]
- 5.Gangji V, Hauzeur JP, Schoutens A, Hinsenkamp M, Appelboom T, Egrise D. Abnormalities in the replicative capacity of osteoblastic cells in the proximal femur of patients with osteonecrosis of the femoral head. J Rheumatol. 2003;30:348-351. [PubMed] [Google Scholar]
- 6.Garg P, Mazur MM, Buck AC, Wandtke ME, Liu J, Ebraheim NA. Prospective Review of Mesenchymal Stem Cells Differentiation into Osteoblasts. Orthop Surg. 2017;9:13-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hauzeur JP, Bernard C, Egrise D, Kurth W, Van Cauwenberge H, Lechanteur C, Gillet P, Beguin Y, Malaise M, Hustinx R. Indium-oxine labelling for evaluating the homing process of autologous osteoblasts implanted percutaneously in atrophic nonunion fractures. Int Orthop. 2013:37:131-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hauzeur JP, Pasteels JL, Orloff S. Bilateral non-traumatic aseptic osteonecrosis in the femoral head. An experimental study of incidence. J Bone Joint Surg Am. 1987;69:1221-1225. [PubMed] [Google Scholar]
- 9.Hernigou P, Beaujean F. Abnormalities in the bone marrow of the iliac crest in patients who have osteonecrosis secondary to corticosteroid therapy or alcohol abuse. J Bone Joint Surg Am . 1997;79:1047-1053. [DOI] [PubMed] [Google Scholar]
- 10.Hernigou P, Beaujean F. Treatment of osteonecrosis with autologous bone marrow grafting. Clin Orthop Relat Res. 2002;405:14-23. [DOI] [PubMed] [Google Scholar]
- 11.Hernigou P, Dubory A, Homma Y, Guissou I, Flouzat Lachaniette CH, Chevallier N, Rouard H. Cell therapy versus simultaneous contralateral decompression in symptomatic corticosteroid osteonecrosis: a thirty year follow-up prospective randomized study of one hundred and twenty five adult patients. Int Orthop. 2018;42:1639-1649. [DOI] [PubMed] [Google Scholar]
- 12.Hernigou P, Flouzat-Lachaniette CH, Delambre J, Poignard A, Allain J, Chevallier N, Rouard H. Osteonecrosis repair with bone marrow cell therapies: state of the clinical art. Bone. 2015;70:102-109. [DOI] [PubMed] [Google Scholar]
- 13.Jensen MP, Chen C, Brugger AM. Interpretation of visual analog scale ratings and change scores: a reanalysis of two clinical trials of postoperative pain. J Pain. 2003;4:407-414. [DOI] [PubMed] [Google Scholar]
- 14.Katz NP, Paillard FC, Ekman E. Determining the clinical importance of treatment benefits for interventions for painful orthopedic conditions. J Orthop Surg Res . 2015;10:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelly AM. The minimum clinically significant difference in visual analogue scale pain score does not differ with severity of pain. Emerg Med J . 2001;18:205-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lalu M, Mcintyre L, Pugliese C, Fergusson D, Winston B, Marshall J, Granton J, Stewart D. Safety of cell therapy with mesenchymal stromal cells (safecell): a systematic review and meta-analysis of clinical trials. Plos One. 2012;7:e47559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mont MA, Cherian JJ, Sierra RJ, Jones LC, Lieberman JR. Nontraumatic osteonecrosis of the femoral head: where do we stand today? A ten-year update. J Bone Joint Surg Am . 2015;97:1604-1627. [DOI] [PubMed] [Google Scholar]
- 18.Mont MA, Marulanda GA, Jones LC, Saleh KJ, Gordon N, Hungerford DS, Steinberg ME. Systematic analysis of classification systems for osteonecrosis of the femoral head. J Bone Joint Surg Am. 2006;88:16-26. [DOI] [PubMed] [Google Scholar]
- 19.Mutijima E, De Maertelaer V, Deprez M, Malaise M, Hauzeur JP. The apoptosis of osteoblasts and osteocytes in femoral head osteonecrosis: its specificity and its distribution. Clin Rheumatol. 2014;33:1791-1795. [DOI] [PubMed] [Google Scholar]
- 20.Piuzzi NS, Chahla J, Schrock J, LaPrade RF, Pascual-Garrido C, Mont MA, Muschler GF. Evidence for the use of cell-based therapy for the treatment of osteonecrosis of the femoral head: A Systematic Review of the literature. J Arthroplasty. 2017;32:1698-1708. [DOI] [PubMed] [Google Scholar]