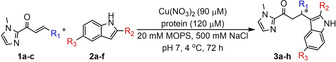
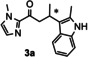
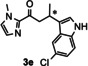
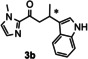
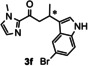
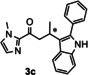
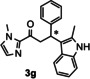
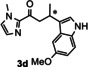
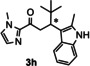
Table 2.
Substrate scope of the vinylogous Friedel‐Crafts alkylation reactions.[a]
|
| |||||
|---|---|---|---|---|---|
|
Entry |
Product |
Y/ee [%][b] Cu2+ Cu2+⊂RamR Cu2+⊂CgmR Cu2+⊂QacR |
Entry |
Product |
Y/ee [%][b] Cu2+ Cu2+⊂RamR Cu2+⊂CgmR Cu2+⊂QacR |
|
1 |
|
5 |
|
||
|
|
22±7/– 57±9/29±3 (−) 52±12/13±3 (−) 78±11/34±3 (−) |
|
<5/n.d. <5/n.d. <5/n.d. 17±8/38±5 (+) |
||
|
2 |
|
6 |
|
||
|
|
7±8/– 5/n.d. 7±3/27±3 (+)−R 27±12/26±2 (+)−R |
|
<5/n.d. <5/n.d. <5/n.d. <5/n.d. |
||
|
3 |
|
7 |
|
||
|
|
<5/n.d. <5/n.d. <5/n.d. <5/n.d. |
|
54±9/– <5/n.d. 18±4/6±2 59±7/75±4 |
||
|
4 |
|
8 |
|
||
|
|
10±6/– 19±3/35±3 (+) 31±14/37±1 (+) 39±10/9±4 (+) |
|
<5/n.d. <5/n.d. <5/n.d. <5/n.d. |
||
[a] Typical conditions: 90 μM Cu(NO3)2 (9 mol%) loading with 1.3 equivalents of protein (120 μM). [b] Yields and ee were determined by HPLC using 2‐phenylquinoline as internal standard. For yields <5 % ee's were not determined. All the results listed correspond to the average of two independent experiments, each of them carried out in duplicate. Errors listed are standard deviations. Signs of optical rotation and absolute configuration were assigned by comparison to the literature.29, 30