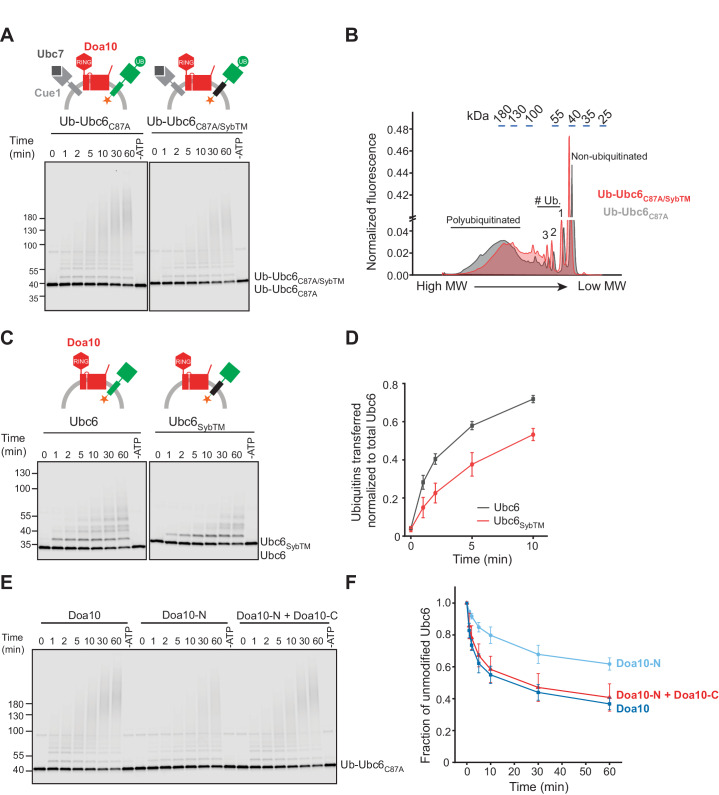
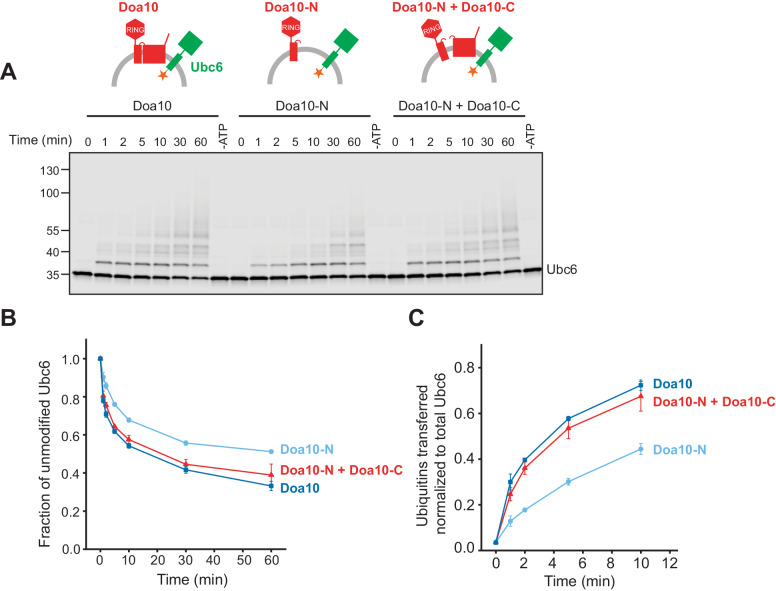
Figure 5. Structural Determinants for Ubiquitination.
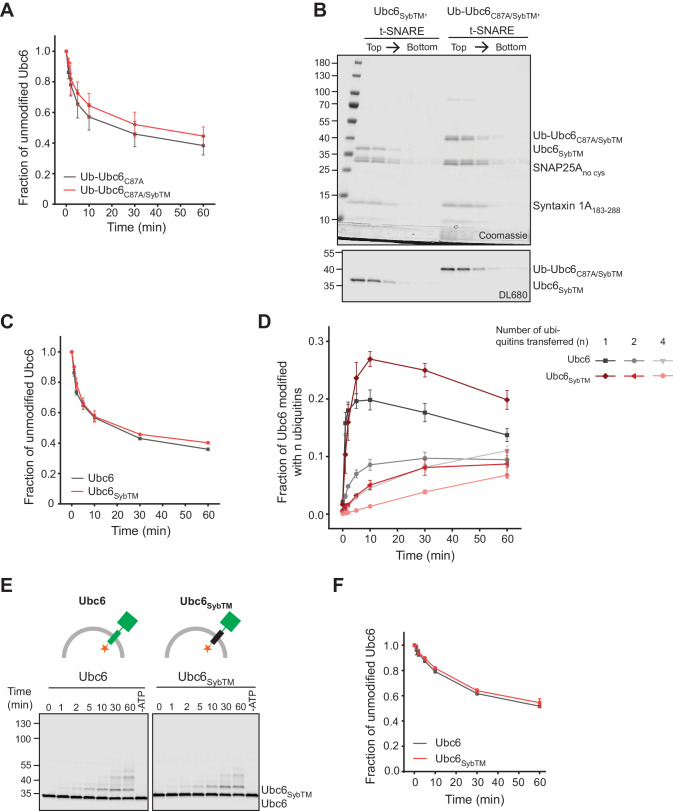
(A) Time course of ubiquitination of Ub-Ubc6C87A or Ub-Ubc6C87A/SybTM by Doa10 in the presence of Cue1/Ubc7. For each reaction, a 60 min sample in the absence of ATP is shown. Samples were analyzed by SDS-PAGE and fluorescence scanning. Final concentrations: 40 nM Doa10, 10 nM Cue1, 1 µM Ubc7, 100 nM Ubc6 variants, 100 nM E1, 120 µM ubiquitin, and 2.5 mM ATP. See Figure 5—figure supplement 1A for quantification of unmodified Ubc6 variants. (B) Comparison of ubiquitin-chain length on Ub-Ubc6C87A or Ub-Ubc6C87A/SybTM. Line-scans were performed on fluorescence images of two representative gel samples (30 min timepoint) as in (A). Approximate molecular weights are indicated on top. # Ub., number of ubiquitin moieties attached. (C) Time-course of Ubc6 WT or Ubc6SybTM ubiquitination in the absence of Ubc7/Cue1. Analysis and concentrations as in (A). See Figure 5—figure supplement 1C for quantification of unmodified Ubc6 variants. (D) Quantification (mean ± SD) of total ubiquitin-transfer to Ubc6 or Ubc6SybTM from three experiments as in (C). Intensities of Ubc6 variants with one to four ubiquitin moieties attached were determined as described in Figure 5—figure supplement 1D, summed up for each time point and normalized to total Ubc6 in the reaction. (E) Time course of ubiquitination of Ub-Ubc6C87A by Doa10 variants in the presence of Cue1/Ubc7. Liposomes contained Ub-Ubc6C87A and either full-length Doa10, only Doa10-N, or both Doa10-N and -C. Analysis and concentrations as in (A). (F) Quantification (mean ± SD) of unmodified Ub-Ubc6C87A from three experiments as in (E).