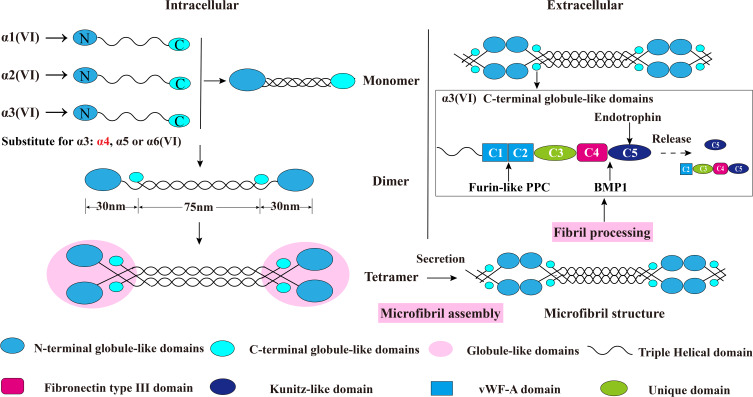
Figure 2.
Schematic diagram of collagen VI assembly process. Collagen VI is firstly assembled into a triple helix monomer by the alpha-1 (VI), alpha-2 (VI) and alpha-3 (VI) chains in a stoichiometric ratio of 1:1:1. Then the triple helix monomers are staggered into anti-parallel dimers bonded by disulfide bonds, and the anti-parallel dimers are also aligned through disulfide bonds to form tetramer. Further, these tetramers are secreted to the extracellular matrix and form beaded microfibrils in a way of end-to-end connection of overlapped N-terminal globular domains through non-covalent bonds. The maturation of collagen VI microfibrils (fibril processing) in the extracellular matrix is a continuous process that starts immediately when tetramers are secreted, and requires proteolytic process involving the removal of the C-terminal domains of the collagen alpha-3 (VI) chain (for example, the C5 or the C2–C5 domains cleavage fragments) after microfibril assembly. The alpha-5 (VI) and alpha-6 (VI) chains can be substituted for alpha-3 (VI) chains in human. Furin-like PPC and BMP-1 are two proteins involving the endotrophin-containing fragments releasing process, and the cleavage sites are between the C4 and C5 domains as well as the the C1 and C2 domains. This figure was modified with permission from Furthmayr H, Wiedemann H, Timpl R, Odermatt E, Engel J. Electron-microscopical approach to a structural model of intima collagen. Biochem J. 1983;211(2):303–311. doi:10.1042/bj2110303. Copyright 1983, Portland Press, Ltd.101
Abbreviations: vWF-A, von Willebrand factor type A; Furin-like PPC, furin-like proprotein convertase; BMP-1, bone morphogenetic protein 1.