Abstract
Background
Transcatheter mitral valve replacement (TMVR) may be a valuable treatment option for mitral annular calcification and severe mitral stenosis (MS) in patients at high operative risk. Pre-procedural virtual and printed simulations may aid in procedure planning, device sizing, and mitigate complications such as valve embolization or left ventricular outflow tract (LVOT) obstruction.
Case summary
We describe a case of TMVR in which multi-detector computed tomography (MDCT) derived, three-dimensional virtual planning and a 3D-printed model of the patients’ left heart provided enhanced understanding of an individual patient’s unique anatomy to determine feasibility, device sizing, and risk stratification. This resulted in deployment of an adequately sized valve. Post-TMVR LVOT obstruction was treated with LVOT balloon dilatation and percutaneous transluminal septal myocardial ablation.
Discussion
Advanced MDCT-derived planning techniques introduce consistent 3D modeling and printing to enhance understanding of intracardiac anatomical relationships and test device implantation. Still, static measurements do not feature haemodynamic factors, tissue, or device characteristics and do not predict device host interaction. Transcatheter mitral valve replacement is feasible in MS when adequately pre-procedurally planned. Multi-detector computed tomography-derived, 3D, virtual and printed models contribute to adequate planning in terms of determining patient eligibility, procedure feasibility, and device sizing. However, static 3D modeling cannot completely eliminate the risk of peri-procedural complications.
Keywords: Transcatheter mitral valve replacement, Pre-procedural planning, 3D virtual models, Medical 3D printing, Case report
Learning points
Transcatheter mitral valve replacement can be performed in high-risk patients with severe mitral stenosis and mitral annular calcification but requires advanced pre-procedural planning.
Pre-procedural planning with multi-detector computed tomography allows generation of three-dimensional virtual and printed models to help define patient eligibility, procedure feasibility, and device sizing.
Static geometrical modeling cannot completely eliminate the risk of peri-procedural complications since it does not integrate haemodynamic and tissue-related factors.
Introduction
Patients with severe mitral annulus calcification (MAC) and stenosis (MS) often have extensive comorbidities and are of high surgical risk.1 Transcatheter mitral valve replacement (TMVR) is a less invasive treatment that precludes decalcification, instead, it exploits annular calcium to facilitate valvular anchoring. However, it comes with additional challenges2,3 making precise pre-procedural planning essential.
We describe a case of TMVR where advanced pre-procedural planning with multi-detector computed tomography (MDCT) provides three-dimensional (3D) virtual modeling and 3D printing to enhance understanding of intracardiac anatomy, device sizing, and allow implantation bench testing.
Timeline
| 2016 | Stroke and detection of moderate–severe mitral stenosis (MS) |
| 2017 | Admission with acute pulmonary oedema with severe pulmonary hypertension and severe MS. Denied conventional mitral valve surgery based on comorbidities |
| 2018–2019 | Recurrent admissions with acute pulmonary oedema despite optimal medical therapy |
| September 2019 | Heart team evaluation consensus for transcatheter mitral valve replacement, creation of a multi- detector computed tomography-derived 3D virtual model |
| October 2019 | Percutaneous coronary intervention of the left anterior descending artery and left circumflex artery in work-up |
| October 2019 | Transcatheter mitral valve replacement complicated by left ventricular outflow tract obstruction which required a percutaneous transluminal septal myocardial ablation |
| Procedure +5 days | Discharge to rehabilitation |
Case presentation
A 65-year-old woman with a medical history of hypertension, diabetes, gout, morbid obesity (body mass index: 41.8 kg/m2), multiple strokes, and coronary artery disease with prior stenting, presented with recurrent acute pulmonary oedema [New York Heart Association (NYHA) functional Class IV] despite optimal medical treatment, among which: metoprolol (1 × 200 mg), intravenous furosemide (2 × 80 mg), dapagliflozine (1 × 10 mg), and spironolactone (1 × 25 mg). Physical examination showed peripheral oedema and orthopnoea in a walking-aid dependent patient. Blood pressure was 138/79 mmHg with a heart rate of 56 b.p.m., a grade II/IV holodiastolic murmur was heard at the 3rd and 4th left intercostal space and bilateral rales were heard upon auscultation of the lungs.
Transoesophageal echocardiography (TOE) revealed severe MAC (Wilkins score 14) and MS (mean gradient 10 mmHg) combined with secondary pulmonary hypertension (>60 mmHg). Basal septum wall thickness was 14 mm with a left ventricular outflow tract (LVOT) mean gradient (MG) of 14 mmHg. The multidisciplinary heart team rejected the patient for surgery due to extensive comorbidities, habitus, poor mobility, and mitral anatomical features and agreed with TMVR. The Society of Thoracic Surgeons predicted risk of mortality score was 5.6%, but further increased by morbid obesity, frailty, and excessive MAC.
Multi-detector computed tomography-derived 3D modeling
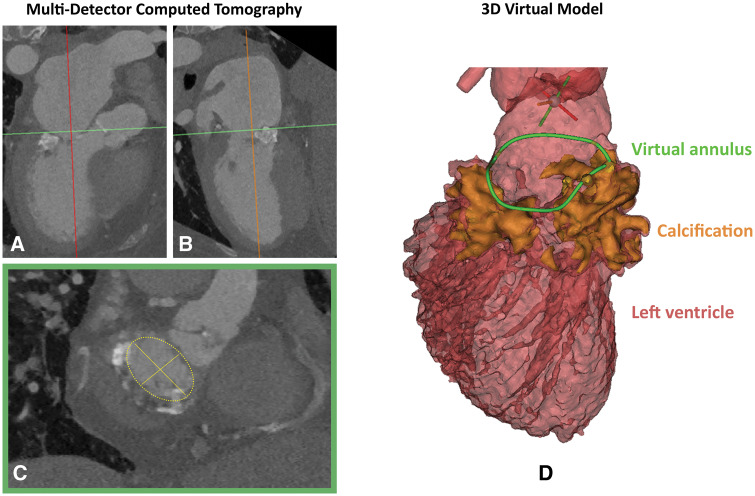
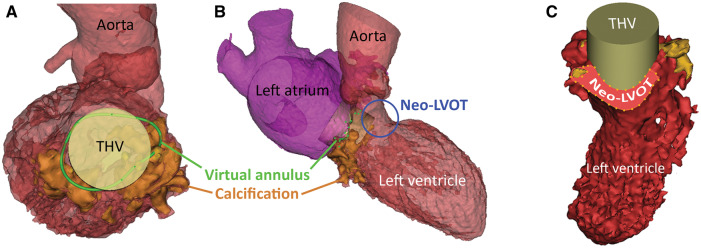
Double oblique MDCT analysis was used to appreciate the extent and distribution of calcifications in the mitral annulus and measure mitral orifice dimensions (Figure 1A–C). Based on these manual measurements a 29 mm (outer diameter) Sapien3 (Edwards Lifesciences Ltd, Irvine, CA, USA) transcatheter heart valve (THV) seemed appropriate. A virtual 3D model was created using the Materialise Mimics™ Enlight medical software package (Materialise, Leuven, Belgium) (Figure 1D). The assessment was derived from the mid-late diastolic phase. Based on the user’s input on the expected landing zone, a best fit plane was obtained to determine minimal and maximal diameters. Transcatheter heart valves of various sizes and at various implantation depths were projected in this plane to determine most suitable device size and estimate the neo-LVOT for each scenario. A 26 mm Sapien3 was deemed the best fit (Figure 2A) positioned 60% in the left atrium and 40% in the left ventricle (Figure 2B and C).
Figure 1.
Multi-detector computed tomography mitral annulus measurement. (A and B) Multi-detector computed tomography image of the left heart, the best fit plane through green line corresponds with the plane of image C (double oblique view). (C) Axial thin section of the mitral annulus. The minimal and maximal diameter were 23.6 mm and 33.4 mm, respectively with an area of 632 mm2 measured manually (D) multi-detector computed tomography-derived, three-dimensional virtual model of the left ventricle and aorta in mid-late diastolic phase. The annular calcium (orange) was used to trace mitral annulus in 3D (green). The minimal and maximal mitral annulus diameters were 22.4 mm and 32.6 mm respectively, with an annular area of 546 mm2.
Figure 2.
Multi-detector computed tomography-derived 3D virtual model of the left heart with a prosthetic heart valve implanted. (A) Same model as in Figure 1D with a cylinder positioned in the mitral annulus representing the dimensions of a 26 mm Sapien3 transcatheter heart valve. (B) Overview of the left heart with the transcatheter heart valve implanted in mitral annulus, note the protrusion into the left ventricular outflow tract forming a neo-left ventricular outflow tract (blue circle). (C) Automatically calculated, the minimal neo-left ventricular outflow tract area in late-systolic phase with an implanted transcatheter heart valve 60% in the left atrium, 40% in the left ventricle was 212 mm2 (30% of original left ventricular outflow tract blocked).
An assessment in the late-systolic phase yielded an acceptable neo-LVOT area of 212 mm2 (30% of original LVOT blocked) (Figure 2C) which was notably higher than suggested cut-off values (>170 mm2).4 Of note, virtual assessment of a Sapien3 29 mm at 60/40 yielded a neo-LVOT of 150 mm2 (53% of original LVOT blocked). A 3D-printed model (By Materialise, Leuven, Belgium) complemented the virtual model and was used to implant an actual Sapien3 26 mm THV (Figure 3A–D) to confirm adequate valve apposition and expansion visually. In this printed model, no evident LVOT obstruction was observed.
Figure 3.
Fitting a transcatheter mitral valve in a life-sized 3D-printed model of the patients’ left heart. Three-dimensional virtual model was 3D-printed on a 1:1 scale. Calcium printed in blue. (A) Surgical view of the left atrium. A Sapien3 26 mm valve on its delivery system is positioned in the mitral annulus. (B) The transcatheter heart valve after deployment in the mitral annulus, adequately fitted. (C) Transcatheter heart valve viewed from the ventricular side. Pliers advanced from the aorta grasp the anterior mitral leaflet, the neo left ventricular outflow tract is highlighted in red. (D) Transcatheter heart valve with deflected anterior mitral leaflet viewed from the aorta shows modest left ventricular outflow tract obstruction (highlighted in red). AML, anterior mitral leaflet; LA, left atrium; LAA, left atrial appendage; LSPV, left superior pulmonary vein; LVOT, left ventricular outflow tract.
Transcatheter mitral valve replacement procedure
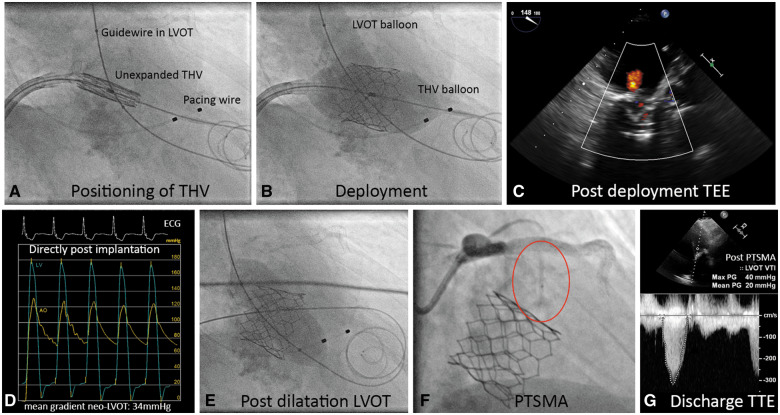
The procedure was performed under general anaesthesia and TOE guidance and femoral access was obtained using a 16-Fr venous and 12-Fr arterial sheath. After transseptal puncture, septostomy was performed with a 12 mm Admiral Xtreme balloon (Medtronic Inc., Minneapolis, MN, USA). A kissing balloon technique was applied that included simultaneous inflation of an 18 mm True balloon (Bard Vascular Inc., Tempe, AZ, USA) in the LVOT while implanting the 26 mm Sapien3 in MAC, aiming for a 60/40 position. The aim of this technique was to preserve a minimum neo-LVOT dimension by pivoting the THV away from the LVOT (Figure 4A and B). Transoesophageal echocardiography confirmed a trace residual paravalvular mitral regurgitation (Figure 4C). The MG was 3 mmHg. However, the neo-LVOT MG increased from 14 mmHg before to 34 mmHg after TMVR (Figure 4D). Balloon dilatation in the neo-LVOT only mildly mitigated the gradient to 28 mmHg (Figure 4E). Percutaneous transluminal septal myocardial ablation (PTSMA) with 2 ml of alcohol to the 1st septal perforator reduced the LVOT gradient to 20 mmHg, measured by transthoracic echocardiogram at discharge (Figure 4F and G). Femoral venous and arterial access were closed with suture based closure devices.
Figure 4.
Transcatheter mitral valve replacement overview. (A) Angiographic image of the unexpanded Sapien3 26 mm transcatheter heart valve positioned in the mitral annulus. A guidewire with 18 mm non-compliant balloon is positioned in the left ventricular outflow tract, a temporary pacemaker wire is placed in the right ventricle. (B) Deployment of the transcatheter heart valve in the mitral annulus with simultaneous inflation of the left ventricular outflow tract balloon under rapid pacing. (C) Post-deployment transoesophageal echocardiography with colour Doppler showing mild mitral regurgitation. (D) Pressure recordings of the left ventricle (blue line) and aorta (yellow line) showing a mean systolic gradient of 34 mmHg. (E) Postdilatation of the left ventricular outflow tract with a 20 mm balloon resulting in a residual mean gradient of 28 mmHg. (F) Percutaneous transluminal septal myocardial ablation in the first septal branch (red circle: 1.5 mm occlusion balloon in the septal perforator branch). (G) Discharge transthoracic echocardiogram showing a residual mean gradient of 20 mmHg over the neo-left ventricular outflow tract. Of note, invasively and transthoracic echocardiogram measured left ventricular outflow tract gradients are not directly comparable.
During the postoperative recovery, the patient developed a total atrioventricular block and required a permanent pacemaker implantation. The remainder of the hospital admission was uneventful and the patient was discharged to a rehabilitation centre on postoperative Day 5. Upon presentation at the outpatient clinic 1 month after discharge, moderate dyspnoea was present (NYHA Class III). No readmission had occurred. Outpatient clinic MDCT evaluation showed a systolic neo-LVOT of 120 mm2, with the THV implanted at a 45/55 position. Re-running the MDCT-derived 3D virtual model with a 26 mm Sapien3 at this implantation height revealed a projected neo-LVOT of 177 mm2.
Discussion
Transcatheter mitral valve replacement is a viable treatment for patients with severe MAC and MS at prohibitive operative risk.2 However, anatomical factors such as location and extent of annular calcification, shape and interaction with surrounding structures pose a risk for serious complications such as valve embolization, paravalvular leakage, or neo-LVOT obstruction.2 Multi-modality pre-procedural planning, is key to assess TMVR eligibility.
Our case description illustrates the added value of MDCT-derived 3D virtual and printed models to refine THV size selection and assure adequate anchoring. The use of a 3D software package resulted in a 26 mm instead of a 29 mm Sapien THV. The 29 mm THV would arguably have resulted in a more dramatic neo-LVOT obstruction. In spite of extensive neo-LVOT assessment in both the virtual and 3D-printed model with estimated neo-LVOT areas well over suggested cut-off values,4,5 the use of the kissing balloon technique and neo-LVOT post-dilatation, there was relevant neo-LVOT obstruction requiring PTSMA. This characterizes the challenge of preventing neo-LVOT obstruction in TMVR, where a tradeoff exists between adequate THV size for anchoring and obstruction of the LVOT. Post-TMVR MDCT analysis showed a smaller than anticipated in vivo neo-LVOT with a THV positioned deeper into the left ventricle than pre-procedurally planned. MDCT-derived 3D models provide a static approximation of the contracting heart in which tissue and device characteristics are currently not well integrated. The posterior distribution of incompressible calcium as well as using a fixed tubular structure to represent the THV may explain the discrepancies found between the predicted and post-TMVR neo-LVOT. Enriching virtual models with tissue and device characteristics as well as haemodynamic factors might add value. Further assessment of the neo-LVOT in a full cardiac cycle could provide more insight in the risk for obstruction.
Conclusion
Transcatheter mitral valve replacement in MS is a challenging procedure which requires careful pre-procedural planning. Advanced MDCT-derived planning techniques introduce consistent 3D modeling and printing to enhance understanding of intra-cardiac anatomical relationships and test device implantation. However, as static geometrical 3D modeling does not incorporate haemodynamic data and tissue or device characteristics, it cannot completely eliminate the risk of peri-procedural complications. Therefore, pre-procedural planning of bail-out strategies remains vital in TMVR.
Lead author biography

Joris Ooms, MD, is a PhD candidate in interventional cardiology at the Thoraxcenter of the Erasmus University Medical Center, Rotterdam, The Netherlands. He is involved in multiple research projects for the structural heart program among which the implementation of advanced pre-procedural planning methods into clinical practice.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Funding
Abbott Vascular International, Boston Scientific, Edwards lifesciences B.V., Medtronic Inc.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The author/s confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: M.M. is employed by Materialise NV. N.V.M. has received research grants from Abbott, Boston Scientific, Edwards, Medtronic, and advisory fees from Abbott, Boston Scientific, Medtronic. The other authors do not have any conflict of interest to declare.
Supplementary Material
References
- 1. Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, Iung B, Lancellotti P, Lansac E, Rodriguez Muñoz D, Rosenhek R, Sjögren J, Tornos Mas P, Vahanian A, Walther T, Wendler O, Windecker S, Zamorano JL; ESC Scientific Document Group. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2017;38:2739–2791. [DOI] [PubMed] [Google Scholar]
- 2. Guerrero M, Urena M, Himbert D, Wang DD, Eleid M, Kodali S, George I, Chakravarty T, Mathur M, Holzhey D, Pershad A, Fang HK, O’Hair D, Jones N, Mahadevan VS, Dumonteil N, Rodes-Cabau J, Piazza N, Ferrari E, Ciaburri D, Nejjari M, DeLago A, Sorajja P, Zahr F, Rajagopal V, Whisenant B, Shah PB, Sinning JM, Witkowski A, Eltchaninoff H, Dvir D, Martin B, Attizzani GF, Gaia D, Nunes NSV, Fassa AA, Kerendi F, Pavlides G, Iyer V, Kaddissi G, Witzke C, Wudel J, Mishkel G, Raybuck B, Wang C, Waksman R, Palacios I, Cribier A, Webb J, Bapat V, Reisman M, Makkar R, Leon M, Rihal C, Vahanian A, O’Neill W, Feldman T.. 1-year outcomes of transcatheter mitral valve replacement in patients with severe mitral annular calcification. J Am Coll Cardiol 2018;71:1841–1853. [DOI] [PubMed] [Google Scholar]
- 3. Guerrero M, Dvir D, Himbert D, Urena M, Eleid M, Wang DD, Greenbaum A, Mahadevan VS, Holzhey D, O’Hair D, Dumonteil N, Rodes-Cabau J, Piazza N, Palma JH, DeLago A, Ferrari E, Witkowski A, Wendler O, Kornowski R, Martinez-Clark P, Ciaburri D, Shemin R, Alnasser S, McAllister D, Bena M, Kerendi F, Pavlides G, Sobrinho JJ, Attizzani GF, George I, Nickenig G, Fassa AA, Cribier A, Bapat V, Feldman T, Rihal C, Vahanian A, Webb J, O’Neill W.. Transcatheter mitral valve replacement in native mitral valve disease with severe mitral annular calcification: results from the first multicenter global registry. JACC Cardiovasc Interv 2016;9:1361–1371. [DOI] [PubMed] [Google Scholar]
- 4. Yoon SH, Bleiziffer S, Latib A, Eschenbach L, Ancona M, Vincent F, Kim WK, Unbehaum A, Asami M, Dhoble A, Silaschi M, Frangieh AH, Veulemans V, Tang GHL, Kuwata S, Rampat R, Schmidt T, Patel AJ, Nicz PFG, Nombela-Franco L, Kini A, Kitamura M, Sharma R, Chakravarty T, Hildick-Smith D, Arnold M, de Brito FS Jr, Jensen C, Jung C, Jilaihawi H, Smalling RW, Maisano F, Kasel AM, Treede H, Kempfert J, Pilgrim T, Kar S, Bapat V, Whisenant BK, Van Belle E, Delgado V, Modine T, Bax JJ, Makkar RR.. Predictors of left ventricular outflow tract obstruction after transcatheter mitral valve replacement. JACC Cardiovasc Interv 2019;12:182–193. [DOI] [PubMed] [Google Scholar]
- 5. Wang DD, Eng MH, Greenbaum AB, Myers E, Forbes M, Karabon P, Pantelic M, Song T, Nadig J, Guerrero M, O'Neill WW.. Validating a prediction modeling tool for left ventricular outflow tract (LVOT) obstruction after transcatheter mitral valve replacement (TMVR). Catheter Cardiovasc Interv 2018;92:379–387. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.