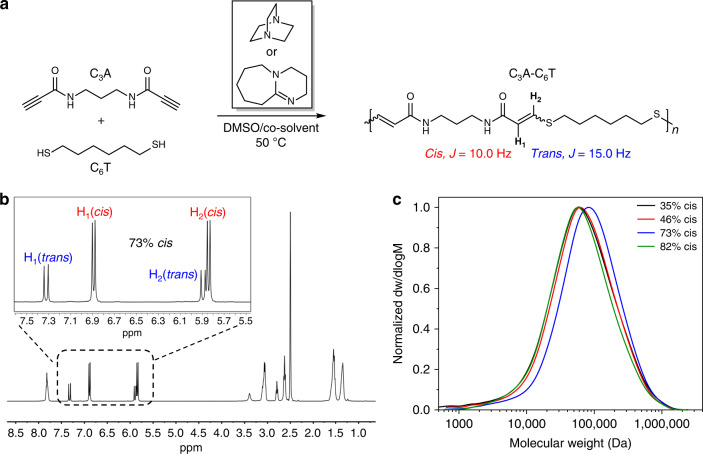
Fig. 1. Characterisation of stereocontrolled polyamides.
a Step-growth polyaddition reaction between C3A and C6T to furnish unsaturated polyamides (C3A-C6T). b Representative 1H NMR spectrum (DMSO–d6, 25 °C, 400 MHz) of C3A-C6T (73% cis content) from using DMSO and DBU. Cis and trans proton resonances were assigned by coupling constants. c SEC (DMF, 0.5 % w/w NH4BF4) chromatograms determined against poly(methyl methacrylate) (PMMA) standards for polyamides with various stereochemistry.