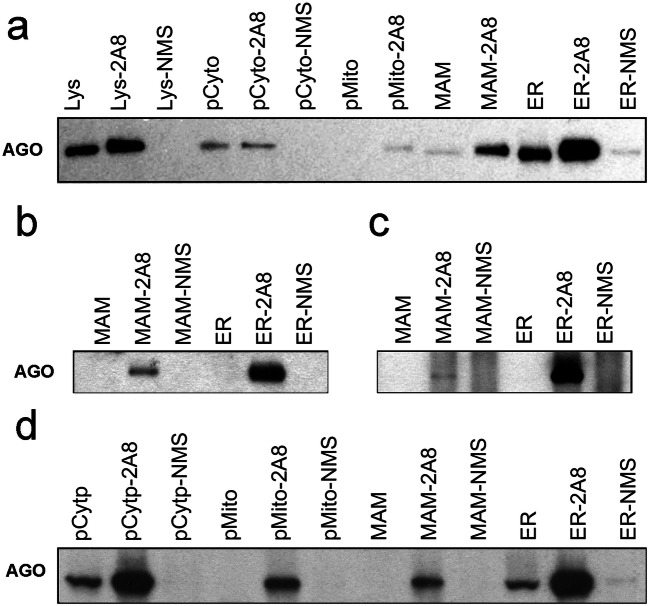
Fig. 6.
Immunoprecipitation and Western blot analysis detection of AGO protein. Human frontal cortex samples from 3 individual cases (a (case 3), b (case 1), and c (case 5)) and rat cortical tissue (d) were subjected to subcellular fractionation followed by immunoprecipitation (IP) with either anti-AGO (2A8, generated in mouse) or normal mouse serum (NMS). The immunoprecipitation products were analyzed by Western blotting using a rabbit anti-AGO antibody (Cell Signaling). AGO was found in all of the 2A8 immunoprecipitation subcellular fractionation samples but not in samples incubated with NMS. Incubation with NMS was not conducted on every mitochondria (pMito) and MAM samples due to the relatively low abundance of protein in these two subcellular fractions. Lys, post-nuclear total lysate; Lys-2A8, post-nuclear total lysate IP with anti-AGO 2A8 antibody; Lys-NMS, post-nuclear total lysate IP with NMS; pCyto, cytosol; pCyto-2A8, cytosol IP with anti-AGO 2A8 antibody; pCyto-NMS, cytosol IP with NMS; pMito, mitochondria lysate; pMito-2A8, mitochondria lysate IP with anti-AGO 2A8 antibody; pMito-NMS, mitochondria lysate IP with NMS; MAM, total MAM lysate; MAM-2A8, MAM lysate IP with anti-AGO 2A8 antibody; MAM-NMS, MAM lysate IP with NMS; ER, total ER lysate; ER-2A8, ER lysate IP with anti-AGO 2A8 antibody; ER-NMS, ER lysate IP with NMS