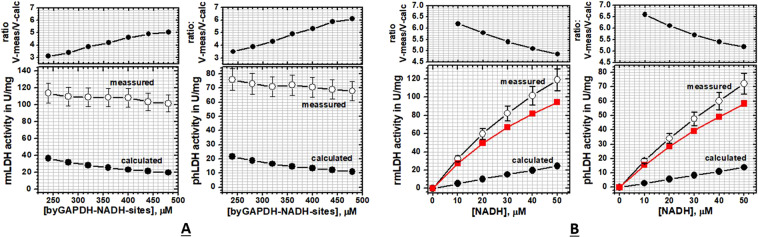
Figure 5.
(A,B) The activity of rabbit muscle LDH and porcine heart LDH was measured in the presence of a large excess of baker´s yeast GAPDH. (A) steady-state activities of rmLDH (10 nM) or phLDH (17 nM) were measured at fixed NADH concentration (40 µM) in the presence of increasing concentration of byGAPDH (240 to 480 μM in terms of NADH binding sites). Increase in byGAPDH concentration leads to the disproportional decrease in measured and calculated free-diffusion activities (lower panels) which results in increase in the ratio between the two activities (upper panels, and Eq. 2-3). These results indicate NADH channeling from byGAPDH-NADH complex to rmLDH or phLDH (Supp. Fig. 11). (B) steady-state activities of rmLDH (10 nM) or phLDH (17 nM) were measured in the presence of decreasing NADH concentration with byGAPDH fixed at 480 μM (NADH binding sites). The decrease in NADH concentration leads to the disproportional decrease in the measured and the calculated free-diffusion activities (lower panels), what results in the increase in the ratio between the two activities (upper panels, and Eq. 2-3). The red curve represents the Michaelis-Menten profile for LDH activity with the byGAPDH-NADH complex as the substrate, which was calculated by subtracting the calculated free-diffusion profile from the measured profiles (Supp. Fig. 14). The calculated apparent KM constant for rmLDH is 78 ± 3 μM and 116 ± 10 μM for phLDH (Table 3). Thus, the observed KM constants are a result of competition between the channeling and free-diffusion paths.