Figure 6.
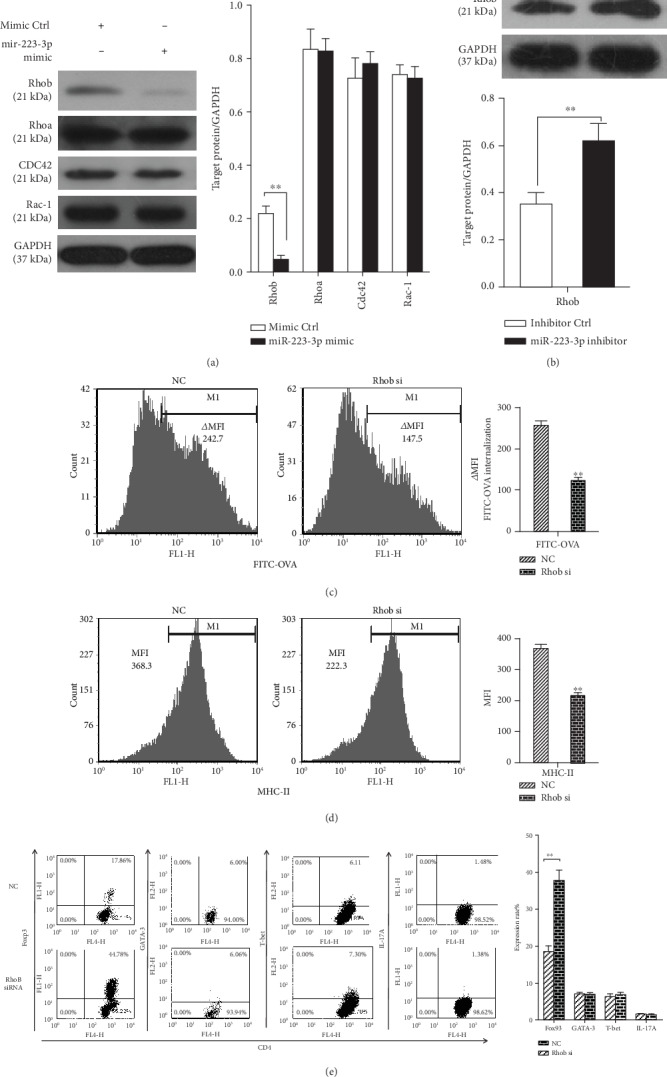
miR-223-3p suppresses Rhob expression to downregulate antigen endocytosis and presentation of BMDCs, followed by Treg cell polarization. (a, b) Mouse immature DCs were transfected with the miRNA mimic control, miR-223-3p mimic, miRNA inhibitor control, and miR-223-3p inhibition, at a final concentration of 50 nM. After 24 h, DCs were stimulated with 100 μg/ml OVA for 24 h. Cell lysates were subjected to Western blot analysis for Rhob, CDC42, Rhoa, and Rac-1 proteins. (c–e) Immature mouse DCs were transfected with 50 nM siRNA-Rhob or siRNA control. (c) After 24 h of transfection, endocytic activity (FITC-OVA uptake) of BMDCs was measured by flow cytometry. (d) After 24 h of transfection, DCs were stimulated with 100 μg/ml OVA for 24 h and then stained with MHC-II antibody, followed by flow cytometry analysis. The bar chart indicated the MFI in the gate of CD11c+ cells in each group. (e) After 24 h of transfection, DCs were stimulated with 100 μg/ml OVA for 24 h. Purified CD4+ T cells were cocultured with the transfected DCs at a ratio of 1 : 10 (DC/T cells). After 4 d, cells were collected and stained with anti-CD4-APC, followed by intracellular staining with FITC-conjugated anti-Foxp3, FITC-conjugated anti-IL-17A, PE-conjugated anti-GATA-3, or PE-conjugated anti-T-bet, respectively. The cells were then analyzed by flow cytometry. The bar chart indicated the specific transcription factor of T cells in the gate of CD4+ cells in each group. Data were presented as mean ± SD of three independent experiments. t-test, ∗P < 0.05, ∗∗P < 0.01.
