Key Points
Question
Is there an efficacious and safe oral treatment for thromboprophylaxis in postoperative patients with suspected gynecologic malignant neoplasms?
Findings
This multicenter randomized clinical trial included 400 women randomized to either oral apixaban or subcutaneous enoxaparin. There were no differences between groups for rates of major bleeding (0.5% vs 0.5%), clinically relevant nonmajor bleeding (5.4% vs 9.7%), and venous thromboembolic events (1.0% vs 1.5%); although adherence rates did not differ, patients in the apixaban group reported increased ease and decreased pain associated with taking the medication.
Meaning
These findings suggest that oral apixaban may offer a safe alternative to subcutaneous enoxaparin that is easier and less painful for patients to take.
This randomized clinical trial investigates the safety and efficacy of oral apixaban vs subcutaneous enoxaparin for postoperative thromboprophylaxis in patients with gynecologic cancer.
Abstract
Importance
Current guidelines recommend a 28-day course of enoxaparin for thromboprophylaxis after surgery for gynecologic cancer. The high cost of this medication and the low adherence rates observed in prior studies provide an opportunity to benefit patients by demonstrating the safety of a more cost-effective, easier to use thromboprophylactic.
Objective
To investigate the safety and efficacy of an oral treatment alternative for thromboprophylaxis in postoperative patients with gynecologic cancer.
Design, Setting, and Participants
This was a patient-based, multicenter, open-label, blinded, end point, randomized clinical trial conducted May 2015 to March 2019 in outpatient and inpatient gynecologic oncology settings. Women undergoing surgery for suspected or confirmed gynecologic cancer were approached for recruitment. The trial compared rates of major bleeding and clinically relevant nonmajor bleeding events during a 90-day follow-up period in patients taking apixaban or enoxaparin for postoperative thromboprophylaxis using a modified intent-to-treat analysis. Data analysis was performed from October to December 2019.
Interventions
Women were randomized to 28 days of apixaban (2.5 mg orally twice daily) or enoxaparin (40 mg subcutaneously daily).
Main Outcomes and Measures
The primary outcome was major bleeding and clinically relevant nonmajor bleeding events. Secondary outcomes included incidence of venous thromboembolic events, adverse events, medication adherence, participant quality of life, and medication satisfaction.
Results
Of 500 women recruited for the study, 400 were enrolled and randomized (median age, 58.0 years; range, 18.0-89.0 years); 204 received apixaban and 196 received enoxaparin. Treatment groups did not differ in terms of race/ethnicity, cancer stage, or surgery modality (open vs robotic). There were no statistically significant differences between the apixaban and enoxaparin groups in terms of rates of major bleeding events (1 patient [0.5%] vs 1 patient [0.5%]; odds ratio [OR], 1.04; 95% CI, 0.07-16.76; P > .99), clinically relevant nonmajor bleeding events (12 patients [5.4%] vs 19 patients [9.7%]; OR, 1.88; 95% CI, 0.87-4.1; P = .11), venous thromboembolic events (2 patients [1.0%] vs 3 patients [1.5%]; OR, 1.57; 95% CI, 0.26-9.50; P = .68), adverse events, medication adherence, or quality of life between the groups. Participant satisfaction was significantly greater in the apixaban group with regard to ease of taking the medication (186 patients [98.9%] vs 110 patients [58.8%]; OR, 0.06; 95% CI, 0.01-0.25; P < .001) and pain associated with taking the medication (4 patients [2.1%] vs 92 patients [49.2%]; OR, 9.20; 95% CI, 2.67-31.82; P < .001).
Conclusions and Relevance
These findings suggest that oral apixaban is a potentially safe, less painful, and easier-to-take alternative to subcutaneous enoxaparin for thromboprophylaxis after surgery for gynecologic cancer. The efficacy of apixaban to prevent venous thromboembolic events is hypothesized as being equivalent.
Trial Registration
ClinicalTrials.gov Identifier: NCT02366871
Introduction
Gynecologic cancers (uterine, ovarian, cervical, and vulvar) affect approximately 100 000 women in the US each year.1 The standard of care for nearly all gynecologic cancers (early and advanced stage) remains aggressive surgical debulking with resection of all visible disease. The extent of surgical resection to microscopic disease remains the factor most significantly associated with overall survival, particularly with ovarian cancer.2
Although specific procedures vary by disease site, nearly all include hysterectomy, removal of adnexa, removal of lymphatic tissue (pelvic and para-aortic), peritoneal biopsies, and omentectomy. These debulking procedures are often aggressive, requiring extended operative times with patients immobile and in a lithotomy position. On average, ovarian cancer debulking procedures may take up to 5 hours to complete.3 In addition, patients with gynecologic cancer tend to have risk factors further predisposing them to surgical morbidity, such as obesity, metabolic syndromes (eg, diabetes, hypertension, and glucose intolerance), and sedentary lifestyles.4
Venous thromboembolism (VTE), which includes deep venous thrombosis (DVT) and pulmonary embolism (PE), remains one of the most lethal complications in women who undergo surgery for gynecologic cancer. Because gynecologic tumors growing within the pelvis involve lymphatic drainage in direct contact with lower extremity vessels, VTE rates are significantly higher in women with gynecologic cancer compared with other cancers.5,6 Rates of DVT after gynecologic cancer have been reported as high as 26% in untreated women and as high as 9% for postoperative PE.7 Pulmonary embolism is associated with mortality, and death may occur in up to 18% to 20% of patients with this complication.8 In addition, there is a risk for major bleeding after gynecologic surgery given the extent of dissection often involved.9
The cost to the health care system of VTE complications is quite high because these patients require extended anticoagulation therapy and surveillance for 6 months after treatment. If a second DVT or PE occurs, lifelong anticoagulation therapy is usually necessary. The extended anticoagulation therapy time adds both financial and resource strain to health care systems because additional monitoring and imaging are required for these women to ensure that VTE is not worsening. The American Society of Clinical Oncology has developed guidelines for postoperative VTE prophylaxis in oncology patients to reduce the health care burden of these complications.10,11 These have been further validated with the adoption of the CHEST 2016 guidelines12 and include the use of preoperative heparin, sequential compression devices during surgery, and postsurgical DVT prophylaxis using heparin and low-molecular-weight heparin. Current recommendations include 28 days of postoperative prophylaxis with low-molecular-weight heparin (enoxaparin 40 mg subcutaneously daily).13
For a variety of reasons, the use of subcutaneous low-molecular-weight heparin has not proven to be ideal. Although the drug has been associated with a decrease in VTE, its use has come into question. Common patient complaints related to low-molecular-weight heparin use include injection site reaction, pain with autoinjection, bruising, bleeding, nausea, vomiting, and cost. Although the rate of adherence to outpatient postoperative thromboprophylaxis in women with gynecologic cancers has not been elucidated, data from the orthopedics literature identified an adherence rate of only approximately 60%.14 With enoxaparin use in outpatient prophylaxis demonstrating poor adherence, a more cost-effective and easier to use medication could affect VTE outcomes. Oral anticoagulation therapy could obviate many of the negative effects associated with the subcutaneous route of administration.
Apixaban, an oral factor Xa antagonist, has been studied in the prevention of VTE for patients undergoing hip and knee replacement surgery.15,16,17 The safety and effectiveness of apixaban compared with enoxaparin and with placebo have been demonstrated as a prophylaxis in surgical patients and in patients for recurrence of VTE. High-risk patients with cancer (approximately 2%) in the ambulatory settings were also assessed.18,19 However, to our knowledge, apixaban has not been investigated for VTE prophylaxis in patients undergoing abdominal surgery with laparotomy or in patients with gynecologic cancers. Given the similar risk profile for postsurgical VTE in patients with gynecologic cancers, the aim of this investigation was to determine the safety (ie, rates of major bleeding and clinically relevant nonmajor [CRNM] bleeding) of apixaban use in women undergoing surgery for suspected gynecologic cancer. Secondary objectives included assessing efficacy in preventing VTE.
Methods
Study Design and Oversight
This trial was a multicenter, prospective, randomized, open-blinded, end point clinical trial conducted at the University of Colorado Hospital in Aurora and University of Southern California, Keck School of Medicine-Hospital in Los Angeles. Because of the difference in mode of delivery (injection vs oral) and the surgical nature of the study population, it was not possible to blind the investigators to the intervention. Patients were approached in the perioperative period, and informed written consent was obtained with institutional review board approval from both institutions. The protocol is outlined in Supplement 1 and the eFigure in Supplement 2. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Per accepted standards, participants were recommended to receive heparin (5000 subcutaneous units) 30 minutes before incision, as well as pneumatic compression devices during surgery and during postoperative hospitalization. Postsurgical care included 5000 subcutaneous units of heparin 3 times per day on the first postoperative day until patients were deemed safe for randomization by the operating surgeon. If epidural anesthesia was used, randomization occurred 8 hours after removal of the catheter. Participants were randomly assigned in a 1:1 ratio to oral apixaban (2.5 mg twice daily) for 28 days or subcutaneous enoxaparin (40 mg daily) for 28 days. Randomization was performed by the inpatient pharmacist at the University of Colorado Hospital, who was impartial to all other aspects of the study, using an online randomizer.20 Patients were evaluated on study day 1 (the day of the first dose), day 14 (±4 days), day 28 (±4 days), and day 90 (±14 days), to assess for the primary and secondary outcomes. Adherence to medication regimen was monitored by participant report, study diary, and assessment of pill bottles or syringes. Participants were deemed adherent with medication if they missed 2 days or fewer of treatment over the course of 28 days (ie, <4 pills or <2 injections missed). The University of Colorado Cancer Center’s Data Safety Monitoring board conducted regular review of the ongoing trial for patient safety and study adherence. An independent blinded reviewer (an oncologist not associated with the trial) conducted assessments of all major and CRNM bleeding events.
Study Population
All patients with a suspected or confirmed diagnosis of gynecologic cancer and who underwent surgery, either by laparotomy or laparoscopy, were eligible for enrollment. Patients with suspected gynecologic cancer included those with a pelvic mass, precancerous lesions of the gynecologic tract, an elevated serum cancer antigen 125 level, and vulvar or cervical lesions. Patients with confirmed gynecologic cancer included those with histologic diagnosis confirmed by pathologic review of an ovarian, uterine, cervical, or vulvar cancer. Women aged 18 to 89 years at 1 of the participating gynecologic cancer centers during the enrollment period who were deemed medically fit for surgery and who were able to return for the 90-day follow-up were included. Race/ethnicity was collected from electronic medical records. This was assessed to be included in the demographic characteristics and to see a broad applicability to the study population. Exclusion criteria included a confirmed pathologic diagnosis other than gynecologic cancer before surgery, known history of VTE, long-term use of nonsteroidal anti-inflammatory drugs, concurrent use of selective serotonin reuptake inhibitors or serotonin-norepinephrine reuptake inhibitors, history of severe renal or hepatic disease, known diagnosis of bleeding dyscrasia (eg, von Willebrand disease), or history of disorders that predispose to hypercoagulability (eg, Factor V Leiden homozygotes or antithrombin deficiency).
Study Outcomes
The primary end point of this study was the incidence of major bleeding and CRNM bleeding events occurring during the treatment phase and in the 30 days after treatment. Secondary end points included incidence of VTE outcomes during treatment and in the 60-day posttreatment period, medication adherence rates, quality of life, and satisfaction of use for oral apixaban compared with subcutaneous enoxaparin.
Major bleeding was defined according to International Society on Thrombosis and Hemostasis criteria,21 including fatal bleeding and/or symptomatic bleeding in a critical area or organ (eg, intracranial, intraspinal, intraocular, retroperitoneal, intra-articular, pericardial, or intramuscular with compartment syndrome) or bleeding requiring the transfusion of 2 units of packed red blood cells. Clinically relevant nonmajor bleeding events were defined as events that did not meet the definition of major bleeding but were associated with medical intervention, unscheduled contact with a physician, temporary cessation of drug therapy, or any other discomfort, such as pain or impairment of activities of daily life.
Screening for VTE was done by the study investigator using the Wells criteria for DVT assessment tool22 at 14, 28, and 90 days after surgery or if standard symptoms developed (eg, unilateral leg swelling, dyspnea, or tachycardia). All participants generally met the criteria for moderate risk by satisfying the Wells criteria of having an active cancer and undergoing major surgery within 4 weeks; therefore, we did not further screen participants only meeting a moderate risk. Participants meeting the criteria for high probability of DVT per the Wells criteria were confirmed by venous Doppler ultrasonography. Determination of suspected PE was measured using additional risk criteria adapted from the Wells criteria modified for PE tool and confirmed by chest computed tomography for those meeting the criteria for high risk.23
Additional secondary analysis included quality of life, compliance, and satisfaction. Quality of life was evaluated using the validated SF-8 health survey provided by Optum,24 which measured overall physical and mental well-being at baseline and end of treatment. Satisfaction was assessed using a participant questionnaire.25 Difficulty remembering to take medication, pain associated with medication, and medication ease of use were measured. Adverse events were graded using the Common Terminology Criteria for Adverse Events system version 4.0.26
Statistical Analysis
A modified intent-to-treat analysis was based on participants initially assigned medication for the first day of treatment. Nonparametric medians tests or t tests were used to compare continuous variables. Fisher exact tests or χ2 tests were used to compare categorical or dichotomous variables. Descriptive statistics including tests for normality for continuous variables were computed. For all values, 2-sided P < .05 was considered statistically significant. SPSS statistical software version 26 (IBM Corp) was used to perform all calculations. Data analysis was performed from October to December 2019.
A previous study27 found an incidence of major bleeding of approximately 5% for the current standard of care (enoxaparin) vs a mean of 3.5% risk of major bleeding associated with apixaban treatment in patients undergoing hip or knee surgery. However, to our knowledge, data are not available for laparotomy or oncologic patients. Given that this population mainly underwent laparotomy, we estimated a slightly increased risk for bleeding in this safety evaluation. Thus, we estimated an 8% rate of major bleeding in the enoxaparin group. With 400 patients enrolled and 80% power with 2-sided α = .05, we would be able to detect a difference of 6 percentage points, or 2% in the apixaban group. This 2% rate is clinically reasonable because we anticipated a higher rate of compliance in that group.
Results
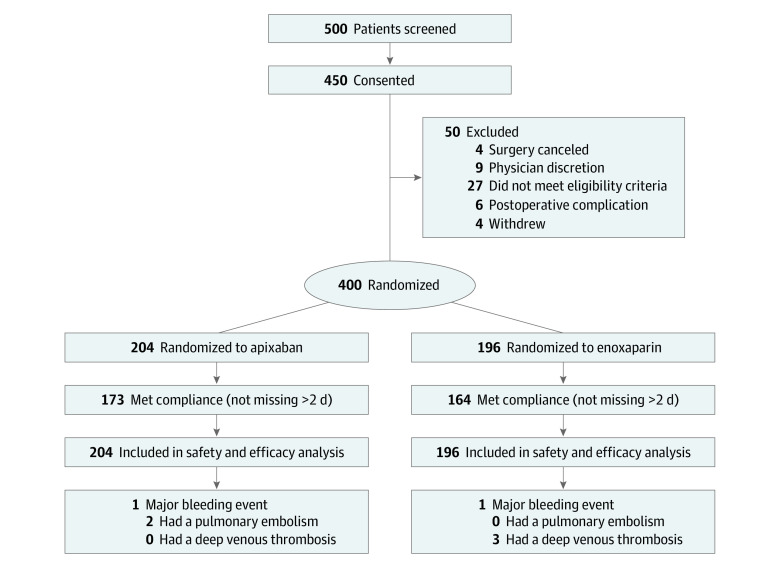
Between May 2015 and March 2019, a total of 500 patients were approached for participation; 50 did not meet the inclusion criteria and 50 consented but failed the screening and did not undergo randomization because of surgeon discretion, patient preference, inability to follow-up, or immediate postoperative complication. Thus, 400 patients were enrolled into the study and included in the final analysis; 204 received apixaban and 196 received enoxaparin (Figure). The median age was 58.0 years (range, 18.0-89.0 years); 322 women (80.5%) were non-Hispanic white, 158 women (39.5%) had high-stage cancer (stages III and IV), and 317 women (79.3%) underwent open vs robotic surgery. The demographic data did not differ between the 2 treatment groups: for the apixaban group vs the enoxaparin group, the median age was 58.0 years (range, 21.0-87.0 years) vs 58.5 years (18.0-89.0 years), 158 patients (77.5%) vs 164 patients (83.7%) were non-Hispanic white, 84 patients (41.2%) vs 74 patients (37.8%) had high-stage cancer, 165 patients (80.9%) vs 152 patients (77.6%) underwent open surgery, 164 patients (80.4%) vs 159 patients (81.1%) had true cancer diagnoses, and the median body mass index (calculated as weight in kilograms divided by height in meters squared) was 27.4 (range, 11.0-50.5) vs 26.5 (range, 15.8-57.2) (Table 1).
Figure. Enrollment, Randomization, and Follow-up.
Flow diagram shows identification of cohort of women who underwent surgical procedures for gynecologic cancers and who were enrolled and treated in the study.
Table 1. Baseline Characteristics of Participants.
| Characteristic | Participants, No. (%) | OR (95% CI) | P value | ||
|---|---|---|---|---|---|
| Apixaban (n = 204) | Enoxaparin (n = 196) | Total (N = 400) | |||
| Age, median (range), y | 58.0 (21.0-87.0) | 58.5 (18.0-89.0) | 58.0 (18.0-89.0) | NA | .30 |
| Body mass index, median (range)a | 27.4 (11.0-50.5) | 26.5 (15.8-57.2) | 27.0 (11.0-57.2) | NA | .26 |
| Race/ethnicity | |||||
| White | 158 (77.5) | 164 (83.7) | 322 (80.5) | NA | .29 |
| Hispanic | 38 (18.6) | 23 (11.7) | 61 (15.3) | ||
| African American | 5 (2.5) | 6 (3.1) | 11 (2.8) | ||
| Asian | 3 (1.5) | 3 (1.5) | 6 (1.5) | ||
| Suspected cancer site | |||||
| Uterine | 81 (39.7) | 83 (42.3) | 164 (41.0) | NA | .94 |
| Ovarian or fallopian | 90 (44.1) | 78 (39.8) | 168 (42.0) | ||
| Cervical | 20 (9.8) | 22 (11.2) | 42 (10.5) | ||
| Vulvar or vaginal | 7 (3.4) | 7 (3.6) | 14 (3.5) | ||
| Other | 6 (2.9) | 6 (3.1) | 12 (3.0) | ||
| Surgical intervention | |||||
| Open | 165 (80.9) | 152 (77.6) | 317 (79.3) | 0.82 (0.50-1.33) | .41 |
| Minimally invasive | 39 (19.1) | 44 (22.4) | 83 (20.8) | ||
| Surgical procedure | |||||
| Total abdominal hysterectomy | |||||
| With bilateral or unilateral salpingo-oophorectomy | 116 (56.9) | 112 (57.1) | 228 (57.0) | 1.01 (0.68-1.50) | .96 |
| Without ovaries | 5 (2.5) | 3 (1.5) | 8 (2.0) | 0.62 (0.15-2.62) | .51 |
| Bilateral or unilateral salpingo-oophorectomy (no total abdominal hysterectomy) | 42 (20.6) | 49 (25.0) | 91 (22.8) | 1.29 (0.81-2.06) | .29 |
| Radical hysterectomy | 18 (8.8) | 24 (12.2) | 42 (10.5) | 1.44 (0.76-2.75) | .26 |
| Lymph node dissection | 93 (45.6) | 88 (44.9) | 181 (45.3) | 0.97 (0.66-1.44) | .89 |
| Bowel resection | 25 (12.3) | 12 (6.1) | 37 (9.3) | 0.47 (0.23-0.96) | .03 |
| Removal of omentum | 82 (40.2) | 82 (41.8) | 164 (41.0) | 1.07 (0.72-1.59) | .74 |
| Surgical complications | 15 (7.4) | 10 (5.1) | 25 (6.3) | 0.67 (0.30-1.54) | .35 |
| Duration of surgery, median (range), min | 204 (31-600) | 215.5 (17-743) | 209 (17-743) | NA | .30 |
| Confirmed diagnosis | |||||
| Malignant or borderline | 164 (80.4) | 159 (81.1) | 323 (80.8) | 1.06 (0.64-1.73) | .83 |
| Benign | 40 (19.6) | 37 (18.8) | 77 (19.3) | ||
| Confirmed site of originb | |||||
| Patients, No. | 164 | 159 | 323 | NA | .43 |
| Uterine | 65 (31.9) | 63 (32.1) | 128 (32.0) | ||
| Ovarian or fallopian | 77 (37.7) | 64 (32.7) | 141 (35.5) | ||
| Cervical | 12 (5.9) | 18 (9.2) | 30 (7.5) | ||
| Vulvar or vaginal | 6 (2.9) | 7 (3.6) | 13 (3.3) | ||
| Other | 4 (2.0) | 7 (3.6) | 11 (2.8) | ||
| Stageb | |||||
| Low (I or II) | 80 (39.2) | 85 (43.4) | 165 (41.3) | 1.14 (0.73-1.76) | .57 |
| High (III or IV) | 84 (41.2) | 74 (37.8) | 158 (39.5) | ||
Abbreviations: NA, not applicable; OR, odds ratio.
Body mass index is calculated as weight in kilograms divided by height in meters squared.
Excludes patients with benign neoplasm.
The attrition rates were 3.8% (15 of 400 patients) at the 28-day study visit and 17.3% (69 of 400 patients) at the 90-day study visit. Attrition occurred as the result of major bleeding (2 patients), VTE (5 patients), medication nonadherence (10 patients), withdrawal from study by the participant (21 patients) or investigator (6 patients), hospitalization (19 patients), hospice admission (1 patient), and death (4 patients). No patient deaths were associated with the study medication, with 2 deaths as a result of sepsis and 2 as a result of disease progression.
Major bleeding occurred in 1 participant in the apixaban group and 1 in the enoxaparin group (0.5% vs 0.5%; odds ratio [OR], 1.04; 95% CI, 0.07-16.76; P > .99) (Table 2). Both patients required blood transfusions, recovered after discontinuation of drug, and had events Common Terminology Criteria for Adverse Events grade 3. Clinically relevant nonmajor bleeding events (hematoma, bruising, epistaxis, and vaginal bleeding) were similar between groups (12 patients in the apixaban group [5.4%] vs 19 patients in the enoxaparin group [9.7%]; OR, 1.88; 95% CI, 0.87-4.1; P = .11) (Table 2). As a composite of both major and CRNM bleeding events, there was no difference between the apixaban and enoxaparin groups (OR, 1.67; 95% CI, 0.81-3.56; P = .16). During the 90-day follow-up period, a total of 11 participants (2.8%) met the high-risk Wells criteria for suspicion of VTE and underwent lower extremity ultrasonography or chest computed tomography angiography. Five participants had VTE (2 in the apixaban group [1.0%] vs 3 in the enoxaparin group [1.5%]; OR, 1.57; 95% CI, 0.26-9.50; P = .68) (Table 2 and eTable 1 in Supplement 2). All participants with confirmed VTE were removed from the study and were prescribed treatment with enoxaparin (1 mg/kg twice daily) for 6 months.
Table 2. Primary and Secondary Outcomes: Major Bleeding, Clinically Relevant Nonmajor Bleeding, and Venous Thromboembolic Events.
| Event | Participants, No. (%) | OR (95% CI) | P value | |
|---|---|---|---|---|
| Apixaban (n = 204) | Enoxaparin (n = 196) | |||
| Major bleeding event | 1 (0.5) | 1 (0.5) | 1.04 (0.07-16.76) | >.99 |
| Clinically relevant nonmajor bleeding events | 12 (5.4) | 19 (9.7) | 1.88 (0.87-4.1) | .11 |
| Hematomaa | 1 (0.5) | 5 (2.6) | 5.31 (0.61-45.9) | .12 |
| Bruisinga | 4 (2.0) | 11 (5.6) | 2.97 (0.93-9.5) | .06 |
| Epistaxis | 3 (1.5) | 2 (1.0) | 0.69 (0.11-4.18) | >.99 |
| Vaginal spotting, discharge, or bleeding | 4 (2.0) | 1 (0.5) | 0.26 (0.03-2.3) | .37 |
| Venous thromboembolism event | 2 (1.0) | 3 (1.5) | 1.57 (0.26-9.50) | .68 |
Abbreviation: OR, odds ratio.
For hematoma and bruising reported, numbers of events were greater than expected.
Adverse events attributable to the study medications were similar between both groups of this investigation with regard to the most common events: for the apixaban group vs the enoxaparin group, 6 patients (2.9%) vs 5 patients (2.6%) were hospitalized within 28 days after surgery, 7 patients (3.4%) vs 10 patients (5.1%) had wound infection, 1 patient (0.5%) vs 5 patients (2.6%) had hematoma, 1 patient each (0.5%) had suspected allergic reaction, 4 patients (2.0%) vs 11 patients (5.6%) had bruising, 7 patients (3.4%) vs 2 patients (1.0%) had arthralgia, 1 patient (0.5%) vs 2 patients (1.0%) had rash, 3 patients (1.5%) vs 2 patients (1.0%) had epistaxis, and 7 patients (3.4%) vs 5 patients (2.6%) had headache (Table 3). More participants in the apixaban group experienced dizziness compared with the enoxaparin group (10 patients [4.9%] vs 1 patient [0.5%]; OR, 0.10; 95% CI, 0.01-0.79; P = .01). There were no grade 4 or 5 adverse events associated with the study treatment (eTable 2 in Supplement 2).
Table 3. Adverse Events.
| Event | Participants, No. (%) | OR (95% CI) | P value | |
|---|---|---|---|---|
| Apixaban (n = 204) | Enoxaparin (n = 196) | |||
| Hospitalized within 28 d after operation | 6 (2.9) | 5 (2.6) | 0.86 (0.26-2.88) | >.99 |
| Wound infection | 7 (3.4) | 10 (5.1) | 1.51 (0.56-4.06) | .41 |
| Dizziness | 10 (4.9) | 1 (0.5) | 0.10 (0.01-0.79) | .01 |
| Suspected allergic reaction | 1 (0.5) | 1 (0.5) | 1.04 (0.07-16.76) | >.99 |
| Arthralgia | 7 (3.4) | 2 (1.0) | 0.29 (0.06-1.41) | .18 |
| Skin rash or cellulitis | 1 (0.5) | 2 (1.0) | 2.1 (0.19-23.3) | .60 |
| Abscess or discharge from incision | 2 (1.0) | 0 | NA | .50 |
| Headache | 7 (3.4) | 5 (2.6) | 0.74 (0.23-2.3) | .77 |
Abbreviation: NA, not applicable; OR, odds ratio.
There were no significant differences in the quality of life SF-8 physical and mental measures in this study, both before and after the intervention, between the 2 groups (median change in physical measures scores, apixaban vs enoxaparin, −5.9 [range, −35.4 to 30.5] vs −6.2 [range, −36.1 to 28.7]; median change in mental measures scores, apixaban vs enoxaparin, 0.8 [range, −30.3 to 30.8] vs 0.0 [range, −30.7 to 41.1]). Participant satisfaction was significantly greater in the apixaban group vs the enoxaparin group with regard to ease of taking medication (186 patients [98.9%] vs 110 patients [58.8%]; OR, 0.06; 95% CI, 0.01-0.25; P < .001) and pain associated with taking the medication (4 patients [2.1%] vs 92 patients [49.2%]; OR, 9.20; 95% CI, 2.67-31.82; P < .001). Participants did not report a difference in difficulty in remembering to take the medication (149 patients [79.3%] vs 149 patients [79.7%] said it was not difficult to remember) (Table 4). There was no significant difference in adherence between the groups, with 173 participants (84.8%) in the apixaban group and 164 participants (83.7%) in the enoxaparin group missing fewer than 2 days of treatment (OR, 0.92; 95% CI, 0.54 to 1.57; P = .76). Nearly all participants completed their 28-day follow-up visit (195 patients [95.6%] in the apixaban group vs 190 patients [96.9%] in the enoxaparin group) (Table 4).
Table 4. Quality of Life, Medication Adherence, and Satisfaction.
| Variable | Apixaban (n = 204) | Enoxaparin (n = 196) | Total (N = 400) | OR (95% CI) | P value |
|---|---|---|---|---|---|
| Quality of life (SF-8) score, median (range) | |||||
| Participants, No. | 176 | 170 | 346 | ||
| Physical | |||||
| Baseline | 47.2 (19.5 to 61.8) | 47.3 (19.9 to 63.1) | 47.2 (19.5 to 63.1) | NA | .96 |
| End of study | 39.2 (21.0 to 58.6) | 38.5 (17.8 to 60.7) | 39.0 (17.8 to 60.7) | NA | .76 |
| Change | −5.9 (−35.4 to 30.5) | −6.2 (−36.1 to 28.7) | −6.0 (−36.1 to 30.5) | NA | .75 |
| Mental | |||||
| Baseline | 50.7 (19.2 to 64.5) | 49.7 (16.2 to 62.7) | 50.1 (16.2 to 64.5) | NA | .64 |
| End of study | 50.7 (18.5 to 69.4) | 49.3 (19.7 to 62.2) | 49.8 (18.5 to 69.4) | NA | .35 |
| Change | 0.8 (−30.3 to 30.8) | 0.0 (−30.7 to 41.1) | 0.0 (−30.7 to 41.1) | NA | .52 |
| Satisfaction survey, participants, No. (%)a | |||||
| Participants, No. | 188 | 187 | 375 | ||
| Difficulty remembering to take medication? | |||||
| Agree | 23 (12.2) | 23 (12.3) | 46 (12.3) | 1.07 (0.43-2.65) | .99 |
| Neutral | 16 (8.5) | 15 (8.0) | 31 (8.3) | 1 [Reference] | |
| Disagree | 149 (79.3) | 149 (79.7) | 298 (79.5) | 1.07 (0.51-2.24) | |
| Pain associated with taking medication? | |||||
| Agree | 4 (2.1)a | 92 (49.2) | 96 (25.7) | 9.20 (2.67-31.82) | <.001 |
| Neutral | 10 (5.3) | 25 (13.4) | 35 (9.4) | 1 [Reference] | |
| Disagree | 173 (92.5) | 70 (37.4) | 243 (65.0) | 0.16 (0.07-0.36) | |
| Was medication easy to take? | |||||
| Agree | 186 (98.9) | 110 (58.8) | 296 (78.9) | 0.06 (0.01-0.25) | <.001 |
| Neutral | 2 (1.1) | 21 (11.2) | 23 (6.1) | 1 [Reference] | |
| Disagree | 0 | 56 (29.9) | 56 (14.9) | NAb | |
| Adherent (missed <2 d) | 173 (84.8) | 164 (83.7) | 337 (84.3) | 0.92 (0.54-1.57) | .76 |
| Completed study visit | |||||
| 28 d | 195 (95.6) | 190 (96.9) | 385 (96.3) | 0.98 (0.14-6.99) | >.99 |
| 90 d | 168 (82.4) | 163 (83.2) | 331 (82.8) | 1.46 (0.24-8.82) | >.99 |
Abbreviations: NA, not applicable; OR, odds ratio.
One patient missed this question.
Odds ratios and 95% CIs cannot be calculated for values of 0.
Discussion
Oral apixaban is a potentially safe alternative with regard to bleeding compared with subcutaneous enoxaparin in women undergoing surgery for gynecologic cancer. There was no significant difference in major or CRNM bleeding events between the 2 groups of this randomized clinical trial. Adherence rates and adverse events between both modalities appeared to be similar, with significantly greater satisfaction outcomes in participants taking apixaban. Although this study was underpowered for the prevention of VTE, rates were slightly lower in the apixaban group, although the difference was not significant. This finding suggests that this modality is effective in preventing VTE with a greater sample size of high-risk women with gynecologic cancer. Since this study started, a retrospective study28 has reported lower VTE rates associated with minimally invasive surgeries. In addition, to our knowledge, this study represents the first prospective trial in which an oral factor Xa inhibitor, apixaban, was safely used in patients undergoing laparotomy. Given the number of cancer operations that require large abdominal incisions in a variety of disease sites (eg, pancreas or colon), these data could be considered in other disease sites that require extensive abdominal debulking.
Previous studies5 have shown the need for anticoagulation in women undergoing surgical debulking of gynecologic cancers. Current recommendations to decrease the risk of VTE have included subcutaneous enoxaparin for 28 days but they have been met with adherence issues because of pain and bruising at injection site and cost.29 Given the potential lethality of VTE in this patient population, effective and convenient prophylaxis is critical in reducing this burden. Our findings suggest that the risk of VTE is still a concern even with appropriate use of anticoagulation drugs and should be considered for the full 28 days after surgery.
Strengths and Limitations
This study has several strengths. First, randomization allowed for potential biases to be substantially reduced and allowed for appropriate balance between the 2 interventions. Both groups had patients with equal numbers disease sites, comorbidities, true cancer diagnoses, and median body mass index. Participants also underwent a variety of surgical procedures, thus increasing the broad applicability of the data. Satisfaction was substantially higher in the apixaban group, with patients stating ease of use as a major factor in adherence.
Notably, the 83.7% rate of adherence to injectables in this study was higher than the rate of 60% reported in prior observational studies14 of patients undergoing orthopedic surgery. In this study, the apixaban adherence rate was 84.8%. Participation in a clinical trial itself may increase the likelihood of adherence. In addition, patients in this study were followed up for 2 weeks postoperatively in person vs by phone and may have been more motivated by serious illness to adhere to medications. We hypothesize that the compliance rates in the real-world setting would reflect the satisfaction we found with taking the medication and that patients taking an oral medication may have higher real-world compliance rates compared with the known real-world compliance rates for those taking enoxaparin daily for a short period.
Limitations of this study include lower than expected rates of VTE and bleeding in the study population, which may be associated with the magnitude of the effect of the primary outcome. Alternatively, the lower than expected VTE rate may be attributed to the high compliance rate in this controlled environment, and it may be difficult to represent the real-world VTE rate. In addition, this study was designed to evaluate the safety outcome of bleeding, and although the data suggest that apixaban is effective, a true randomized noninferiority trial would have to be executed to confirm these findings. This study was also limited in that we could not effectively blind the patients to the study drug without compromising the minimal and justified risk to the patient because of the 2 drastically different study drug administration methods.
Conclusions
These findings suggest that oral apixaban is a safe potential alternative to subcutaneous enoxaparin for thromboprophylaxis in women undergoing surgery for gynecologic cancer. Satisfaction measures were markedly better in the apixaban group, and adherence rates were similar between both modalities in the controlled environment of this trial. Surgeons should continue to use appropriate postoperative VTE prophylaxis in high-risk surgical oncology patients to help prevent this potentially life-threatening outcome and may consider at their discretion the safety of thromboprophylaxis options.
Trial Protocol
eTable 1. Description of Major Bleeding and Venous Thromboembolic Events
eTable 2. Grade Level of Related and Anticipated Adverse Events
eFigure. Treatment Diagram
Data Sharing Statement
References
- 1.American Cancer Society . Cancer facts and figures 2014. Published 2014. Accessed June 1, 2020. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2014.html
- 2.Martín-Cameán M, Delgado-Sánchez E, Piñera A, Diestro MD, De Santiago J, Zapardiel I. The role of surgery in advanced epithelial ovarian cancer. Ecancermedicalscience. 2016;10:666. doi: 10.3332/ecancer.2016.666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crawford SC, Vasey PA, Paul J, Hay A, Davis JA, Kaye SB. Does aggressive surgery only benefit patients with less advanced ovarian cancer? results from an international comparison within the SCOTROC-1 Trial. J Clin Oncol. 2005;23(34):8802-8811. doi: 10.1200/JCO.2005.02.1287 [DOI] [PubMed] [Google Scholar]
- 4.Kumar A, Bakkum-Gamez JN, Weaver AL, McGree ME, Cliby WA. Impact of obesity on surgical and oncologic outcomes in ovarian cancer. Gynecol Oncol. 2014;135(1):19-24. doi: 10.1016/j.ygyno.2014.07.103 [DOI] [PubMed] [Google Scholar]
- 5.Mokri B, Mariani A, Heit JA, et al. Incidence and predictors of venous thromboembolism after debulking surgery for epithelial ovarian cancer. Int J Gynecol Cancer. 2013;23(9):1684-1691. doi: 10.1097/IGC.0b013e3182a80aa7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abu Saadeh F, Norris L, O’Toole S, Gleeson N. Venous thromboembolism in ovarian cancer: incidence, risk factors and impact on survival. Eur J Obstet Gynecol Reprod Biol. 2013;170(1):214-218. doi: 10.1016/j.ejogrb.2013.06.004 [DOI] [PubMed] [Google Scholar]
- 7.Clarke-Pearson DL, Abaid LN. Prevention of venous thromboembolic events after gynecologic surgery. Obstet Gynecol. 2012;119(1):155-167. doi: 10.1097/AOG.0b013e31823d389e [DOI] [PubMed] [Google Scholar]
- 8.Konstantinides SV, Vicaut E, Danays T, et al. Impact of thrombolytic therapy on the long-term outcome of intermediate-risk pulmonary embolism. J Am Coll Cardiol. 2017;69(12):1536-1544. doi: 10.1016/j.jacc.2016.12.039 [DOI] [PubMed] [Google Scholar]
- 9.Watrowski R, Jäger C, Forster J. Improvement of perioperative outcomes in major gynecological and gynecologic-oncological surgery with hemostatic gelatin-thrombin matrix. In Vivo. 2017;31(2):251-258. doi: 10.21873/invivo.11053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lyman GH, Khorana AA, Falanga A, et al. ; American Society of Clinical Oncology . American Society of Clinical Oncology guideline: recommendations for venous thromboembolism prophylaxis and treatment in patients with cancer. J Clin Oncol. 2007;25(34):5490-5505. doi: 10.1200/JCO.2007.14.1283 [DOI] [PubMed] [Google Scholar]
- 11.Key NS, Khorana AA, Kuderer NM, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol. 2020;38(5):496-520. doi: 10.1200/JCO.19.01461 [DOI] [PubMed] [Google Scholar]
- 12.Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315-352. doi: 10.1016/j.chest.2015.11.026 [DOI] [PubMed] [Google Scholar]
- 13.Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schuunemann HJ. Executive summary: antithrombotic therapy and prevention of thrombosis, 9th ed. American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2)(suppl):7S-47S. doi: 10.1378/chest.1412S3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colwell CW Jr, Pulido P, Hardwick ME, Morris BA. Patient compliance with outpatient prophylaxis: an observational study. Orthopedics. 2005;28(2):143-147. doi: 10.3928/0147-7447-20050201-16 [DOI] [PubMed] [Google Scholar]
- 15.Lassen MR, Raskob GE, Gallus A, Pineo G, Chen D, Portman RJ. Apixaban or enoxaparin for thromboprophylaxis after knee replacement. N Engl J Med. 2009;361(6):594-604. doi: 10.1056/NEJMoa0810773 [DOI] [PubMed] [Google Scholar]
- 16.Lassen MR, Raskob GE, Gallus A, Pineo G, Chen D, Hornick P; ADVANCE-2 Investigators . Apixaban versus enoxaparin for thromboprophylaxis after knee replacement (ADVANCE-2): a randomised double-blind trial. Lancet. 2010;375(9717):807-815. doi: 10.1016/S0140-6736(09)62125-5 [DOI] [PubMed] [Google Scholar]
- 17.Lassen MR, Gallus A, Raskob GE, Pineo G, Chen D, Ramirez LM; ADVANCE-3 Investigators . Apixaban versus enoxaparin for thromboprophylaxis after hip replacement. N Engl J Med. 2010;363(26):2487-2498. doi: 10.1056/NEJMoa1006885 [DOI] [PubMed] [Google Scholar]
- 18.Agnelli G, Buller HR, Cohen A, et al. ; AMPLIFY Investigators . Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369(9):799-808. doi: 10.1056/NEJMoa1302507 [DOI] [PubMed] [Google Scholar]
- 19.Agnelli G, Buller HR, Cohen A, et al. ; AMPLIFY-EXT Investigators . Apixaban for prevention of venous thromboembolism. N Engl J Med. 2013;368(8):699-708. doi: 10.1056/NEJMoa1207541 [DOI] [PubMed] [Google Scholar]
- 20.Diem K. Scientific Tables. 6th ed. Geigy; 1962. [Google Scholar]
- 21.Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis . Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3(4):692-694. doi: 10.1111/j.1538-7836.2005.01204.x [DOI] [PubMed] [Google Scholar]
- 22.Wells PS. Advances in the diagnosis of venous thromboembolism. J Thromb Thrombolysis. 2006;21(1):31-40. doi: 10.1007/s11239-006-5573-x [DOI] [PubMed] [Google Scholar]
- 23.Wells PS, Anderson DR, Rodger M, et al. Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D-dimer. Thromb Haemost. 2000;83(3):416-420. doi: 10.1055/s-0037-1613830 [DOI] [PubMed] [Google Scholar]
- 24.Optum . SF-8 health survey. Accessed June 1, 2020. https://www.optum.com/solutions/life-sciences/answer-research/patient-insights/sf-health-surveys/sf-8-health-survey.html
- 25.QualityMetric . Health outcomes scoring software 5.1, version 5.12017. Published 2016. Accessed June 1, 2020. https://www.amihealthy.com/download/installationguide_scoringsoftwarev5.pdf
- 26.Cancer Therapy Evaluation Program . Common Terminology Criteria for Adverse Events (CTCAE), version 4.02010. Updated March 27, 2020. Accessed June 1, 2020. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm
- 27.Lassen MR, Gallus A, Raskob GE, Pineo G, Chen D, Ramirez LM; ADVANCE-3 Investigators . Apixaban versus enoxaparin for thromboprophylaxis after hip replacement. N Engl J Med. 2010;363(26):2487-2498. doi: 10.1056/NEJMoa1006885 [DOI] [PubMed] [Google Scholar]
- 28.Graul A, Latif N, Zhang X, et al. Incidence of venous thromboembolism by type of gynecologic malignancy and surgical modality in the National Surgical Quality Improvement Program. Int J Gynecol Cancer. 2017;27(3):581-587. doi: 10.1097/IGC.0000000000000912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gómez-Outes A, Terleira-Fernández AI, Lecumberri R, Suárez-Gea ML, Vargas-Castrillón E. Direct oral anticoagulants in the treatment of acute venous thromboembolism: a systematic review and meta-analysis. Thromb Res. 2014;134(4):774-782. doi: 10.1016/j.thromres.2014.06.020 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Description of Major Bleeding and Venous Thromboembolic Events
eTable 2. Grade Level of Related and Anticipated Adverse Events
eFigure. Treatment Diagram
Data Sharing Statement