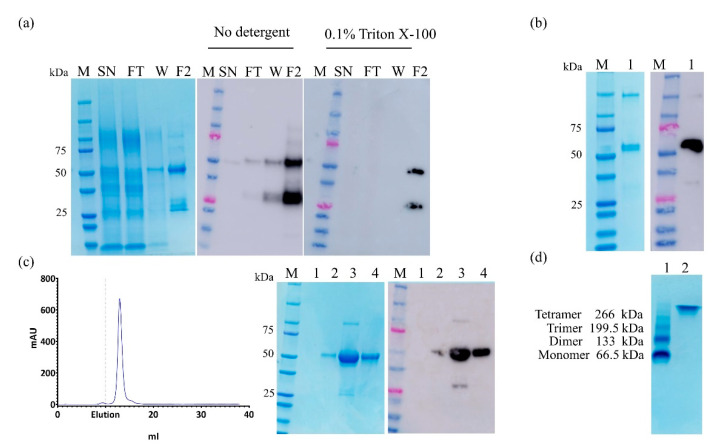
Figure 2.
Purification of plant produced ML-II. (a) Purification of ML-II KDEL on immobilized metal ion affinity chromatography (IMAC). Fractions were analyzed by SDS-PAGE and Western blot. Lane 1, M protein molecular weight marker; Lane 2, clarified crude extract (SN); Lane 3, flow-through (FT) of proteins applied to the TALON column; Lanes 4, TALON resin wash fractions (W); Lanes 5, elution fractions with and without addition of detergent; (b) Second step purification by anti-FLAG M2 affinity resin. Lane 1, purified ML-II analyzed by SDS-PAGE and Western blot. The SDS-PAGE gel shows two bands, and the whole ML-II protein and the higher molecular band were confirmed to be mostly ML-II by LC/MS; (c) Purification of ML-II by gel filtration using Superdex™ 75 10/300GL column at pH 7.4 (PBS). The chromatogram of the elution profile of the ML-II protein was recorded at 280 nm. The beginning of the elution is indicated by dotted lines. Fractions 1, 2, 3, and 4 were analyzed by SDS-PAGE and Western blot, as well as native-PAGE; (d) Lane 1, bovine serum albumin (BSA) was used as a positive control with the respective sizes of a monomer, dimer, trimer, and tetramer. Lane 2, purified ML-II.