Table 2.
Opioid Receptor Activity Measured in the Forskolin-induced cAMP Accumulation Assay a.
| Compound | Structure | MOR cAMP Agonist Potency ± SEM (nM) (% Efficacy) |
MOR Mediated β-arrestin Recruitment (% Control, Emax DAMGO), nM | Bias Factor | DOR cAMP Agonist Potency ± SEM (nM) (% Efficacy) |
KOR cAMP Agonist Potency ± SEM (nM) (% Efficacy) |
|---|---|---|---|---|---|---|
| Group 1. N-Cyanoalkyl Compounds and Compounds Lacking Substituents on the Aromatic Ring | ||||||
| 1a |
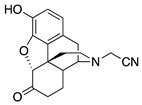
|
4.16 ± 0.64 (101.6 ± 1.1) |
424 ± 69 (33.4 ± 0.25) |
2.18 | 254 ± 167 (77 ± 4) |
109.4 ± 45.4 (99.3 ± 4.0) |
| 1c |
|
76.7 ± 7.4 (103 ± 0.9) |
584 ± 286 (4.1 ± 0.1) |
1.35 | 1699 ± 847 (96.5 ± 5.4) |
>10,000 |
| 1d |
|
18.29 ± 4.70 (95.2 ± 1.2) |
130.4 ± 37.5 (1.9 ± 0.4) |
2.53 | 596 ± 92 (94.4 ± 1.4) |
>10,000 |
|
S5
Hydromorphone |
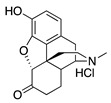
|
1.67 ± 0.30 (102 ± 1) |
159 ± 28 (19.8 ± 3) |
3.48 | 134.3 ± 31.4 (95.4 ± 0.8) |
176.1 ± 32.7 (80.4 ± 3.9) |
|
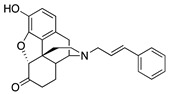
S11
N-Phenethylnor- hydromorphone |
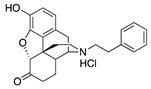
|
0.04 ± 0.01 (102.1 ± 0.8) |
1.71 ± 0.66 (61.9 ± 5.2) |
0.47 | 1.54 ± 0.22 (95.1 ± 0.8) |
22.7 ± 4.8 (80.6 ± 18.3) |
| Group 2. Nitro Substituents | ||||||
| 2a |
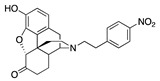
|
0.05 ± 0.02 (102 ± 0.4) |
6.38 ± 0.59 (26.2 ± 2.8) |
4.51 | 0.53 ± 0.03 (96 ± 1.5) |
74.6 ± 5.6 (63 ± 1.5) |
| 2b |
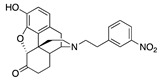
|
5.22 ± 1.20 (102.3 ± 0.6) |
116 ± 21 (6 ± 0.1) |
3.38 | 46.7 ± 17.6 (95.3 ± 1.3) |
>10,000 |
| Group 3. Alkyl and Alkoxy Substituents | ||||||
| 2c |
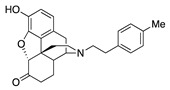
|
0.08 ± 0.02 (101.5 ± 0.2) |
5.6 ± 1.4 (46.5 ± 3) |
1.12 | 1.0 ± 0.3 (95.0 ± 1.3) |
8.71 ± 0.09 (99.4 ± 0.8) |
| 2d |
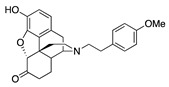
|
0.13 ± 0.04 (103 ± 2) |
11.2 ± 1.8 (23 ± 2.2) |
2.69 | 2.71 ± 0.93 (97.5 ± 0.7) |
7.38 ± 2.10 (96.2 ± 1.0) |
| Group 4. Halides and Trifluromethyl Substituents | ||||||
| 2e |
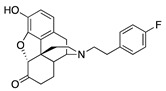
|
0.17 ± 0.08 (100.8 ± 1.1) |
15.5 ± 2.1 (45.8 ± 2.4) |
1.39 | 2.58 ± 1.60 (98.1 ± 1.1) |
19.9 ± 1.3 (80.0 ± 3.8) |
| 2f |
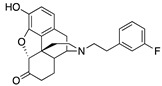
|
0.06 ± 0.01 (101.7 ± 0.3) |
6.2 ± 1.1 (40.3 ± 2.3) |
1.94 | 1.64 ± 0.11 (96.9 ± 1.6) |
56.5 ± 21.1 (58.0 ± 8.4) |
| 2g |
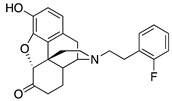
|
0.010 ± 0.003 (101.2 ± 0.1) |
1.7 ± 0.6 (73.2 ± 2.1) |
1.37 | 0.26 ± 0.13 (93.8 ± 2.1) |
19.9 ± 0.6 (74.0 ± 5.2) |
| 2h |
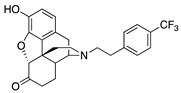
|
0.21 ± 0.04 (102 ± 0.2) |
10.3 ± 1.8 (62.4 ± 1.5) |
0.56 | 2.41 ± 0.47 (94.3 ± 1.8) |
98.2 ± 7.7 (95.7 ± 0.6) |
| 2i |
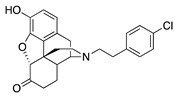
|
0.05 ± 0.03 (98.8 ± 2.1) |
2.44 ± 0.45 (44.8 ± 1.8) |
0.82 | 0.53 ± 0.18 (84.8 ±5.6) |
55.2 ± 26.1 (76.6 ± 11.3) |
| 2j |
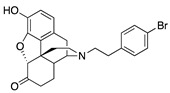
|
0.15 ± 0.04 (103 ± 1.5) |
4.9 ± 0.3 (53 ± 0.4) |
0.43 | 0.97 ± 0.22 (96 ± 1.1) |
20.1 ± 3.3 (99.04 ± 3.74) |
| 2k |
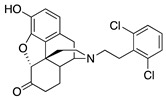
|
0.37 ± 0.09 (101.3 ± 0.5) |
13.13 ± 0.19 (24.6 ± 3.9) |
1.04 | 21.89 ± 0.87 (95.8 ±1.1) |
1881 ± 687 (73.97 ± 5.49) |
| Standards | ||||||
| - | DAMGO | 0.3 ± 0.04 (101.5 ± 0.5) |
44.1 ± 3.9 (103 ± 0.4) |
1.0 | - | |
| - | U-69593 | - | - | - | - | 0.7 ± 0.3 (101.2 ± 1.4) |
| - | Leu-Enkephalin | - | - | - | 0.04 ± 0.10 (95 ± 2) |
- |
| - | Morphine | 4.7 ± 0.6 (102.9 ± 0.6) |
378 ± 41 (25.7 ± 0.6) |
2.27 | - | - |
a Inhibition of forskolin-induced cAMP accumulation; DAMGO (([D-Ala2, N-MePhe4, Gly-ol]-enkephalin); U-69593 ((+)-(5α,7α,8β)-N-methyl-N-[7-(1-pyrrolidinyl)-1-oxaspiro[4.5]dec-8-yl]-benzeneacetamide); Leu-Enkephalin (Tyr-Gly-Gly-Phe-Leu). To determine % efficacy of MOR-mediated β-arrestin recruitment, the highest dose(s) of DAMGO were used as 100% and the respective data were converted to percentages based on the response of DAMGO and analyzed using GraphPad Prism. To determine % efficacy in forskolin-induced cAMP assays, data were normalized to the vehicle control, followed by the forskolin control. Data were then analyzed in GraphPad Prism using nonlinear regression.
