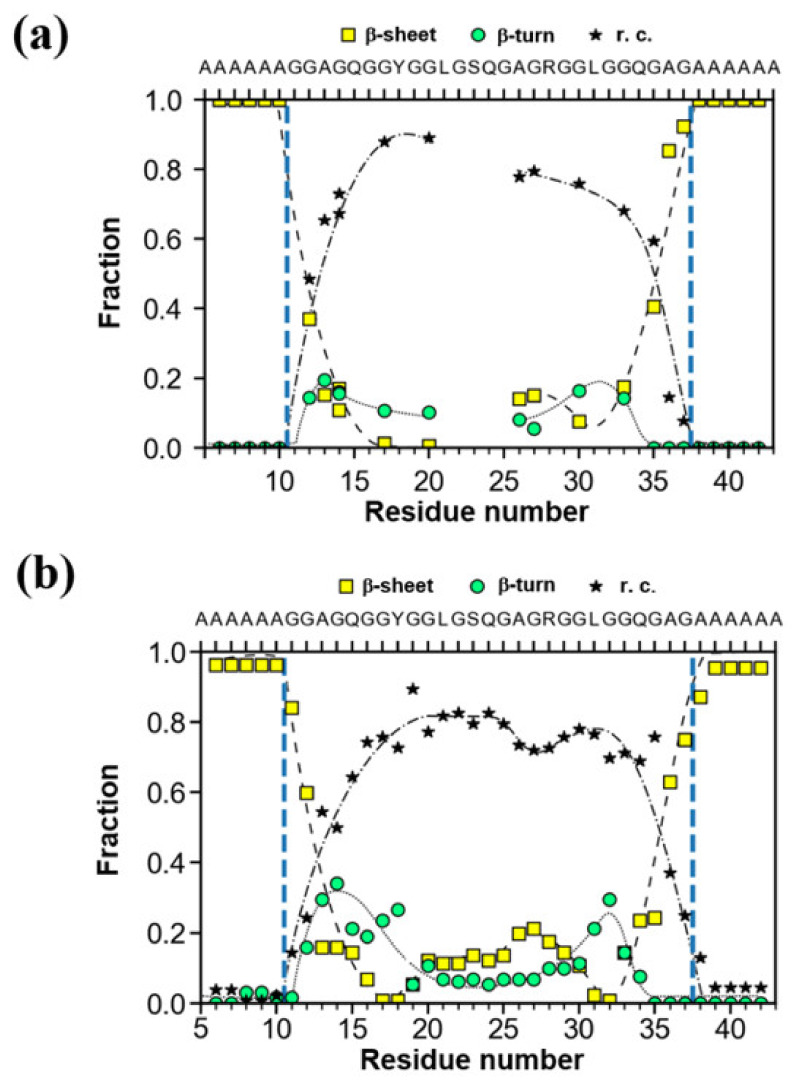
Figure 6.
(a) The fractions of several conformations, i.e., AP-β sheet, random coil and β-turn determined using 13C CP/MAS NMR for the 13C-labeled individual residues in 47-mer peptide, (E)6(A)6GGAGQGGYGGLGSQGAGRGGLGGQGAG(A)6(E)6 (Table 1 (I)) as the sequential model of the Gly-rich region (Figure 3a). The broken, dash-dotted and solid lines indicate change in the fractions of AP-β sheet, random coil and β-turn conformations, respectively as a function of residue number. (b) The fractions of several conformations calculated by the structural identification (STRIDE) algorithm, for the representative structure of the replica exchange molecular dynamics (REMD) simulations with the lowest temperature replica for the individual residues in the sequence, (A)6GGAGQGGYGGLGSQGAGRGGLGGQGAG(A)6 shown for a comparison [62]. The arrangement of the peptide chains with the sequences before MD simulation was as follows. The four extended peptide chains were oriented in antiparallel forms within the planes and parallel between the planes. Three parallel planes were assumed. The intermolecular hydrogen bonding systems of the (A)6 blocks were set in a staggered packing arrangement.