Abstract
OBJECTIVE:
To determine the outcomes and mechanisms of delayed low-intensity extracorporeal shock wave therapy (Li-ESWT) in a rat model of irreversible SUI.
MATERIALS AND METHODS:
Twenty-four female Sprague-Dawley rats were randomly assigned into 3 groups: sham control, vaginal balloon dilation (VBD)+β-aminopropionitrile (BAPN) (SUI group), and VBD + BAPN +treatment with Li-ESWT (SUI-Li-ESWT group). An irreversible SUI model was developed by inhibiting the urethral structural recovery with BAPN daily for 5 weeks. Thereafter, in the SUI-Li-ESWT group, Li-ESWT was administered twice per week for 2 weeks. After a 1-week washout, all 24 rats were evaluated with functional and histological studies at 17 weeks of age. Endogenous progenitor cells were detected via the EdU-labeling method.
RESULTS:
Functional analysis with leak point pressure (LPP) testing showed that the SUI-Li-ESWT group had significantly higher LPPs compared to untreated rats. Increased urethral and vaginal smooth and striated muscle content and increased thickness of the vaginal wall were noted in the SUI-Li-ESWT group. The SUI group had significantly decreased neuronal nitric oxide (nNOS)/ tyrosine hydroxylase (TH) positive nerves ratio in the smooth muscle layers of the urethra, while the SUI-Li-ESWT group had nNOS/TH+ nerves ratio similar to that of the control group. The continuality of urothelial cell lining was also improved in the SUI-Li-ESWT group. In addition, there were significantly increased EdU-positive cells in the SUI-Li-ESWT group.
CONCLUSION:
Li-ESWT appears to increase smooth muscle content in the urethra and the vagina, increase the thickness of urethral wall, improve striated muscle content and neuromuscular junctions, restore the integrity of the urothelium, and increase the number of EdU-retaining progenitor cells in the urethral wall.
Keywords: Low-intensity extracorporeal shock wave therapy (Li-ESWT), stress urinary incontinence (SUI), vaginal balloon dilation (VBD), β-aminopropionitrile (BAPN), leak-point pressure (LPP), 5-ethynyl-2-deoxyuridine (EdU)
The worldwide incidence of stress urinary incontinence (SUI) was projected to be 167 million people (a prevalence of 3.3%) by 2018 1, 2. Current non-surgical treatment options used include pelvic floor exercises, electrical stimulation, biofeedback, hormone creams, and pessaries. Although most of these therapies are safe, their efficacy and durability are limited. Surgical treatments for SUI include injection of bulking agents along with various modifications of bladder neck suspension procedures and mid-urethral tapes. Mesh surgeries for SUI can be very effective, but unfortunately many patients experience complications that include infection, mesh erosion, pelvic pain, and dyspareunia. These problems resulted in more than 140,000 lawsuits in the United States alone and led to total bans of using mesh for pelvic organ prolapse (POP) in the United States 3, Australia, and the United Kingdom (UK). Mesh surgeries for SUI was also halted in the UK in July 2018.
Increased awareness of mesh-related complications has propelled interest in applying regenerative medicine methods for restoring urethral sphincter and pelvic floor structure and function 4,5. The medical and surgical options currently available for SUI provide variable benefit for patients [5], and most of these treatment options provide only symptomatic relief [6]. Novel treatments for SUI are needed to correct the underlying pathology and to cure the symptoms. To this end, stem cell therapy currently dominates the research field for SUI 6-8 and tissue engineering with stem cells dominates the research field for POP 9. There is also increasing interest in exploiting stem cell products and tissue-resident stem/progenitor cells 10 to improve the regeneration process. Despite the overwhelming enthusiasm of scientists, clinicians, and patients alike, stem cell therapy for SUI nevertheless requires considerable investigation prior to its disseminated clinical implementation.
The most important risk factors for SUI in women are vaginal delivery and menopause 11. Previously, we established an animal model for female SUI 12. By prolonged vaginal distension and oophorectomy, the rats recapitulate the pathology of childbirth-induced tissue injury and provide a good model for the study of SUI. However, after 1 month many rats regain continence spontaneously, making this an undesirable model for the study of long-term SUI. Recently, we modified the animal model by administering β-aminopropionitrile (BAPN) to inhibit lysyl oxidase, an enzyme that is critical in the crosslinking of collagen and elastin. This impairs the biogenesis of collagen and elastin fibers and creates a highly reproducible model of irreversible SUI 13.
Low-intensity extracorporeal shock wave therapy (Li-ESWT) has been used to treat musculoskeletal disorders for decades 14. Recent studies showed that Li-ESWT has been expanded to address neurovascular erectile dysfunction 15. Li-ESWT has the ability to mobilize endogenous stem cells to the injury site 16, promote nerve regeneration 15, and reverse muscle content reduction 15, 16. In our previous study, we treated an SUI animal model with Li-ESWT shortly after vaginal dilation and discovered significant preventative effects17. Most SUI in women occurred years after childbirth, however. Therefore, this current study was designed to treat the animals in a delayed fashion, long after SUI developed, to determine the therapeutic effects. To explore whether or not delayed treatment with Li-ESWT could generate results similar to our earlier study, we combined our previous methods to establish a stable, irreversible SUI animal model, and we tested the therapeutic effects and possible mechanisms of Li-ESWT at two dosage levels.
MATERIALS AND METHODS
Experimental Animals and Design
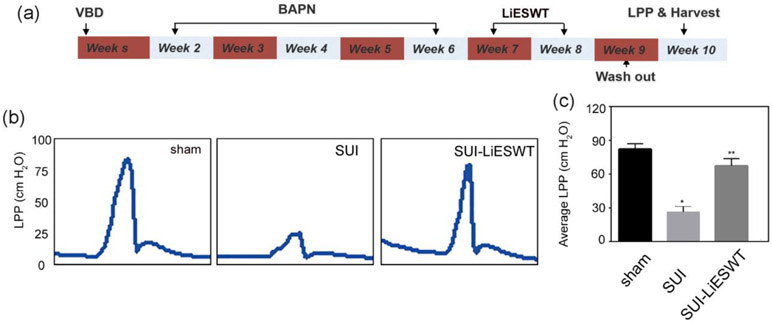
All the experiments were approved by the Institutional Animal Care and Use Committee at the University of California, San Francisco. Twenty-four female Sprague-Dawley rats (Charles River Laboratories; Wilmington, MA; USA) were injected with 5-ethynyl-2-deoxyuridine (EdU) (Invitrogen; Carlsbad, CA; USA) (50mg/kg, intraperitoneally) at birth for detection of label-retaining cells (LRCs) as previously described18. At 8 weeks of age, these animals were randomly divided into 3 groups including the control group (Sham), vaginal balloon dilation (VBD) + BAPN (SUI group), VBD + BAPN +treatment with Li-ESWT wave twice a week (SUI-Li-ESWT group). The VBD procedure and subsequent oophorectomy were performed as previously reported 13. Briefly, under proper anesthesia (intraperitoneal ketamine/xylazine, 90mg/kg and 10mg/kg, respectively), the balloon of an 18Fr latex Foley catheter was inserted into the rat’s vagina, with subsequent filling of 4 mL water. A 130-g weight was placed on the suspended end of the catheter, providing a constant pull to direct the force to the pelvic floor. The balloon was left in place for 4 hours. Three days later, the rats were anesthetized again, and bilateral ovaries were removed. From the second week after initial VBD, all the VBD rats received intraperitoneal injections of 300 mg/kg of BAPN twice a week for 5 weeks. After 2 weeks of Li-ESWT treatment (during week 7 and 8) and 1 week of wash-out, all rats underwent leak-point pressures (LPP) measurement followed by excision of the urethra and vagina for histological study before euthanization (Fig 1a).
Figure 1. Experimental design of the delayed Li-ESWT therapy experiment.
(a) Experimental protocol from week 1 to week 10; (b) Representative graphs of LPP (cm H2O) measurement among the 3 groups: sham, SUI, and SUI-Li-ESWT; (c) Average LPP of the 3 groups. (n=8 * p < 0.05, compared with the sham group ** 0.01 < p < 0.05, compared with the SUI group.)
Low-Intensity Extracorporeal Shock Wave (Li-ESWT)
Li-ESWT was performed by using the MTS Dermagold device (Atlanta, GA; USA). The shockwave probe was applied directly to the shaved skin over the lower pelvis of the rat, aiming at the urethra. The probe was coupled to the skin using ultrasound gel (Aquasonic, Parker Laboratories Inc, Fairfield, NJ; USA) with energy flux density (EFD) 0.06mJ/mm2, 300 pulses at 3 Hz. Rats were treated with Li-ESWT twice a week for two weeks.
Measurement of Leak Point Pressure
Leak point pressure (LPP) was assessed as previously described 19. Briefly, under anesthesia with urethane, a polyethylene-90 tube was inserted into the bladder dome and secured with a purse string suture. The bladder was slowly filled with warmed phosphate buffered saline (PBS), and the volume was recorded. Bladder capacity was recorded as the point at which leakage of urine was observed. This procedure was repeated 3 times, and the average bladder capacity was obtained. The bladder was subsequently emptied via aspiration and manual pressure. Intravesical pressure changes were recorded by a computer with LabView 6.0 software (National Instruments, Austin, TX, USA) at a sample rate of 10 samples/sec. The bladder was then filled to 40% of capacity and increasing manual extravesical pressure was applied until leakage was noted. This procedure was repeated six times, and the LPPs were recorded. The rats were subsequently sacrificed, and tissues harvested.
Phalloidin and α-Bungarotoxin Staining
For muscle staining, tissue sections were incubated with Alexa-488/594-conjugated phalloidin (1:500; Invitrogen, Carlsbad, CA, USA) diluted 1:200 in 1% bovine serum albumin for 20 min at room temperature. After rinsing with PBS, the tissues were stained with 4’,6-diamidino-2-phenylindole (DAPI, 1 μg/ml; Sigma-Aldrich; St. Louis, MO; USA) for nuclear staining. Neuromuscular junctions were stained with α-bungarotoxin (α-BTX, 1:500 Invitrogen) followed by phalloidin.
Immunofluorescence and EdU Staining
Urethral cryosections were prepared, and immunofluorescence was performed as previously described. In brief, tissues were rehydrated with PBS for 5 min and treated with hydrogen peroxide/methanol to quench endogenous peroxidase activity. After rinsing, sections were washed twice in PBS for 5 min followed by 30 min of incubation with 3% horse serum/PBS/0.3% triton X-100. After excess fluid was drained, sections were incubated overnight at 4°C with primary antibodies including anti-neuronal nitric oxide synthase (nNOS; 1:200; Santa Cruz Biotechnologies, Santa Cruz, CA; USA), anti-tyrosine hydroxylase (TH; 1:200; Millipore Corp, Bedford, MA; USA), anti-α-smooth muscle (α-SMA) antibody (1:1000; Sigma-Aldrich, St. Louis, MO; USA), and anti- myosin heavy chain (MHC; 1:500; Abcam, Cambridge, MA; USA). Secondary antibodies used included Alexa-488- and Alexa-594-conjugated antibodies (1:500; Invitrogen). Nuclei were stained with 4’, 6-diamidino-2-phenylindole (DAPI). Sections were assessed for EdU-labeling using the Click-iT reaction cocktail (Invitrogen), which contained Alexa Fluor 594 (1:500), for 30 minutes at room temperature followed by nuclear staining with DAPI (1 pg/ml, Sigma-Aldrich). For image analysis, four randomly selected fields per tissue per animal for each group were photographed and recorded using a Retiga Q Image digital still camera and ACT-1 software (Nikon Instruments Inc., Melville, NY, USA). For assessment of EdU- labeled cells, the entire urethral section of each rat was surveyed, and the number of EdU-labeled cells was counted.
Statistical Analysis
Results were analyzed using GraphPad Prism Version 5.0 (GraphPad Software, San Diego, CA, USA) and expressed as mean ± standard error of the mean. Statistical analyses were performed using one-way analysis of variance followed by the Tukey’s post hoc test for multiple comparisons. Differences among groups were considered significant at P < 0.05.
RESULTS
Delayed Treatment with Li-ESWT Improved LPP in the SUI Group
The beneficial effects of Li-ESWT on LPP are illustrated in Figure 1b. The sham group exhibited a normal LPP whereas the SUI group (VBD + oophorectomy + BAPN) exhibited decreased LPP. In contrast, the peak LPPs were improved in the Li-ESWT treatment group (SUI-Li-ESWT group) (Figure 1c). No significant difference was found in the bladder capacity among the three groups.
Muscular Damage in SUI Rats was reversed by Li-ESWT
The overall content of both urethral striated and smooth muscle and vaginal wall smooth muscle in the SUI group was lower than observed in the sham group (Figure 2 & sFigure 1). Li-ESWT treatment significantly increased the number and length of urethral striated muscle fibers. In addition, Li-ESWT increased the content of smooth muscle in both the urethra and the vaginal wall. The vaginal wall was significantly thinner in the SUI group (185±47um) as compared to the control group (353±45um). Li-ESWT significantly reversed this pathology in the SUI-Li-ESWT group (304±59um) (P<0.05).
Figure 2. Li-ESWT ameliorated the decline of muscular structure.
(a) Middle section of urethra presents urethral striated muscle (MHC, green) and urethral smooth muscle (a-SMA, red). Upper panel: co-localization of urethral striate muscle and smooth muscle (x40); lower panel: urethral striate muscle and smooth muscle at high magnification (x400); (b) Data were presented as number of urethral striated muscle fibers per high power field. (c) Data were presented as length (um) of urethral striated muscle fibers. N=8, * p < 0.05 compared with the sham group, ** p < 0.05 compared with the SUI group.
Effects of Li-ESWT on nNOS+ and TH+ Nerve Fibers
Urethral nitrergic nerve content was examined with nNOS staining and sympathetic nerve content was checked with TH staining (sFigure 2). In the urethra of the sham control rats, both nNOS+ and TH+ nerves appeared sporadically in the smooth muscle layer, and the ratio of nNOS/TH was 0.8. In the SUI group, the ratio decreased to 0.2 with a significant decrease of nNOS+ nerve fibers and a large increase of TH+ nerves. Li-ESWT reversed the ratio to approximately 1:1 (sFigure 2) with an increase of nNOS+ nerve fibers and a decrease of TH+ nerve fibers.
Delayed Li-ESWT Reversed the Decrease of Urethral Neuromuscular Junctions (NMJs) in SUI-Li-ESWT Rats
The neuromuscular junction (NMJ) is the key structure for innervation of urethral striated muscle. Urethral striated muscle is essential for sphincter contractility. With α-BTX staining, we noted that rats in the SUI group had decreased NMJ content as compared to rats in the sham group (Figure 3). After Li-ESWT, rats in the SUI-Li-ESWT group had a significant increase in urethra NMJs.
Figure 3. Li-ESWT increased formation of neuromuscular junctions (NMJ).
(a) Representative staining of NMJs with a-BTX (green) and phalloidin (red) among three groups. (b) Total number of NMJs in each cross-section of urethra. * p < 0.05 compared with the sham group, ** p < 0.05 compared with the SUI group.
Li-ESWT Increased the Number of EdU-Positive Progenitor Cells in the Urethra
Progenitor cells, or cells with stem properties, are characterized by the ability to retain EdU for a prolonged period of time. Therefore, we examined the EdU-positive cells to identify and monitor the progenitor cells in the urethra. After inducing SUI, the number of EdU-positive cells increased in the SUI group as compared to the sham group. Li-ESWT enhanced this effect. The distribution of EdU+ cells was different among these groups (Figure 4). In the SUI group, increased EdU+ cells were noted in the striated muscle layer. In the SUI-Li-ESWT treated group, more EdU+ cells were present in the mucosal layer and the smooth muscle layer.
Figure 4. Li-ESWT increased recruitment of EdU+ label-retaining cells (LRCs).
(A) Representative staining of EdU and phalloidin among the groups. (B) Total number of EdU+ cells was increased after Li-ESWT; (C) The distribution of EdU+ cells was changed after Li-ESWT treatment, * p < 0.05, ** 0.01 < p < 0.05, *** p <0.01 compared with the sham group.
DISCUSSION
In this study, we demonstrated that Li-ESWT has multiple significant effects on the structure and function of the urethra in treated rats with irreversible stress incontinence induced by VBD, oophorectomy, and BAPN. Li-ESWT increased the number of EdU+ progenitor cells, reversed the decrease in muscle content, down-regulated TH expression, and increased the number of nNOS+ nerve fibers. Li-ESWT also enhanced neuromuscular junction regeneration. These changes were associated with a physiologically relevant increase in leak point pressure. These results strongly indicate that Li-ESWT is capable of restoring urinary continence in rats after VBD, oophorectomy, and BAPN.
Decades ago, our team developed the first animal model to study the mechanisms of SUI associated with childbirth by using a vaginal distension method 20. This model has been modified in multiple different ways, but most animals recover continence within 4-6 weeks 21, 22. Because many patients with SUI are parous and post-menopausal, we later added oophorectomy to VBD23. BAPN has been used extensively to study the contribution of the extracellular matrix to normal tissue function in various disease states. BAPN interferes with the synthesis of collagen and elastin fibers by inhibiting lysyl oxidase 24. Our previous work introduced BAPN into VBD treated rats to produce a consistent model of long-term irreversible SUI 13. Of the women who have remission of SUI after delivery, more than half re-develop SUI 5 years later 25. Thus, in the present study, we combined the VBD, oophorectomy, and BAPN to create a reproducible and clinically relevant long-term irreversible SUI animal model.
While conventional ESWT in the field of urology is primarily utilized to treat stone diseases, in recent years Li-ESWT is been used as an emerging therapy for erectile dysfunction 15, 26. Our previous study showed that Li-ESWT can activate resident stem/progenitor cells, Schwann cells, and endothelial cells to enhance tissue regeneration. Recently, Zissler et al. reported that Li-ESWT can stimulate regeneration of skeletal muscle tissue and accelerate repair processes after acute muscle injury27. In addition, we previously demonstrated that Li-ESWT shortly after VBD can prevent incontinence by protecting the urethral sphincter. In the clinical setting, it is impractical to treat every woman with Li-ESWT shortly after delivery. With this in mind, our study includes a 5-week interval between the initial VBD procedure and the Li-ESWT treatment. Our data suggest that Li-ESWT can be a therapeutic modality to reverse tissue damage and increase LPP in SUI rats.
Anatomically, the urinary continence control system consists of two parts: the urethral sphincteric closure system and the urethral supportive system28. The sphincteric closure of the urethra is provided by urethral striated muscle, urethral smooth muscle, and submucosal urethral vascular elements. SUI can be caused by multiple factors, but urethral sphincter deficiency is likely responsible for most cases of SUI 29, especially in women with history of vaginal delivery11. Compatible with our understanding of human female SUI, our present study observed decreased muscle content in the urethra and vaginal wall of the animal model. After Li-ESWT treatment, the total amount of muscle was dramatically restored.
The normal function of the urinary continence control system depends not only on the integrity of the urethral anatomy but also on the coordination between nerves and the muscular structures. In our SUI model, we were surprised to find that the number of TH+ sympathetic nerve fibers was much higher in the SUI group than in the control group. Moreover, after Li-ESWT, the upregulated sympathetic nerves were decreased in the SUI-Li-ESWT group. The Th+ sympathetic nerve fibers were mainly distributed within the longitudinal smooth muscle.This muscle shortens the urethra and decreases urethral resistance when activated 30. We speculate that hyperactive sympathetic nerves in the SUI group may decrease LPP by shortening the functional urethral length and that Li-ESWT restores urinary function via inhibiting the growth of TH+ nerves. Moreover, increased nNOS innervation after Li-ESWT may help to increase urethral compliance and facilitate sphincteric closure by striated muscle during physical exertion. In addition, the contractility of urethral striated muscle is controlled by somatic cholinergic nerves which connect with muscle fibers via NMJs. We noted that Li-ESWT significantly enhanced the formation of NMJs, which helps to re-innervate the regenerated muscle fibers.
The mechanisms of Li-ESWT for SUI remain unclear. Activation and recruitment of sufficient progenitor cells to the target organ is usually the first step of tissue regeneration. In this study, we used the Edu label-retaining technique and identified more progenitor cells in the urethra tissue after induction of SUI than in the sham group. This is consistent with the process of tissue repair after injury. We found even more progenitor cells in all layers of the urethra after treatment with Li-ESWT, which may account for the therapeutic effects of low dose Li-ESWT.
Atrophy of the vaginal wall after oophorectomy or menopause in women is a well-known condition. Our SUI group clearly showed decreases in smooth muscle content and thickness of the vaginal wall. Li-ESWT restored vaginal smooth muscle content and vaginal wall thickness without estrogen therapy. This suggests that Li-ESWT may ameliorate urethral hypermobility in women by restoring the structure and function of the vaginal wall, which provides support of the urethra. On the other side, the age dependent loss of progenitor cells and age dependent loss of muscle satellite cells are very critical for the therapeutic outcome 31,32,33, more studies are needed.
A major limitation of the current study is that we did not investigate the exact mechanisms of interaction between Li-ESWT and tissue. We are also aware that the age dependent loss of progenitor cells and muscle satellite cells may negatively affect the therapeutic outcome 31,32,33, and more studies in older animals are needed. Additional studies are also needed to determine the fate of cells after in-situ activation by Li-ESWT. We hope to collaborate with both physicists and clinicians in forthcoming studies to delve deeper into the basis of Li-ESWT’s biological effects in order to determine whether Li-ESWT may be a viable therapeutic option for SUI.
CONCLUSION
By combining VBD, oophorectomy, and BAPN, we created a novel animal model of prolonged irreversible SUI, mimicking the human condition after childbirth trauma and menopause. We found that delayed treatment with Li-ESWT can restore both the functional and structural defects that contribute to SUI. We suspect that these improvements are mediated via activation of EdU+ stem/progenitor cells, regeneration of urethral and vaginal muscles, inhibition of TH+ nerves, and amplification of NMJs. Our results suggest that Li-ESWT is a non-invasive modality to facilitate tissue regeneration and improve voiding function in an animal model of SUI. Thus, it may provide a novel, non-invasive therapeutic approach for SUI patients in the future.
Supplementary Material
Acknowledgments:
Research reported in this publication was supported by NIDDK of the National Institutes of Health under award number R56DK105097 and 1R01DK105097-01A1. Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by and do not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCE:
- 1.D'Angelo W, Dziki J, Badylak SF. The challenge of stress incontinence and pelvic organ prolapse: revisiting biologic mesh materials. Current opinion in urology. 2019;29:437–42. [DOI] [PubMed] [Google Scholar]
- 2.Irwin DE, Kopp ZS, Agatep B, Milsom I, Abrams P. Worldwide prevalence estimates of lower urinary tract symptoms, overactive bladder, urinary incontinence and bladder outlet obstruction. BJU international. 2011;108:1132–1138. [DOI] [PubMed] [Google Scholar]
- 3.FDA. Urogynecologic Surgical Mesh Implants 2019,April 16. [Google Scholar]
- 4.Bennington J, Williams JK, Andersson KE. New concepts in regenerative medicine approaches to the treatment of female stress urinary incontinence. Current opinion in urology. 2019. [DOI] [PubMed] [Google Scholar]
- 5.Vinarov A, Atala A, Yoo J, et al. Cell therapy for stress urinary incontinence: Present-day frontiers. J Tissue Eng Regen Med. 2018;12:e1108–e1121. [DOI] [PubMed] [Google Scholar]
- 6.Lin CS, Lue TF. Stem cell therapy for stress urinary incontinence: a critical review. Stem cells and development. 2012;21:834–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gill BC, Damaser MS, Vasavada SP, Goldman HB. Stress incontinence in the era of regenerative medicine: reviewing the importance of the pudendal nerve. The Journal of urology. 2013;190:22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thaker H, Sharma AK. Regenerative medicine based applications to combat stress urinary incontinence. World J Stem Cells. 2013;5:112–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chapple CR, Osman NI, Mangera A, et al. Application of Tissue Engineering to Pelvic Organ Prolapse and Stress Urinary Incontinence. Low Urin Tract Symptoms. 2015;7:63–70. [DOI] [PubMed] [Google Scholar]
- 10.Blau HM, Daley GQ. Stem Cells in the Treatment of Disease. N Engl J Med. 2019;380:1748–1760. [DOI] [PubMed] [Google Scholar]
- 11.Chai TC, Asfaw TS, Baker JE, et al. Future Directions of Research and Care for Urinary Incontinence: Findings from the National Institute of Diabetes and Digestive and Kidney Diseases Summit on Urinary Incontinence Clinical Research in Women. The Journal of urology. 2017;198:22–29. [DOI] [PubMed] [Google Scholar]
- 12.Breyer BN, Wang G, Lin G, et al. The effect of long-term hormonal treatment on voiding patterns during filling cystometry and on urethral histology in a postpartum, ovariectomized female rat. BJU international. 2010;106:1775–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang G, Lin G, Zhang H, et al. Effects of prolonged vaginal distension and beta-aminopropionitrile on urinary continence and urethral structure. Urology. 2011;78:968 e913–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ioppolo F, Rompe JD, Furia JP, Cacchio A. Clinical application of shock wave therapy (SWT) in musculoskeletal disorders. Eur J Phys Rehabil Med. 2014;50:217–230. [PubMed] [Google Scholar]
- 15.Li H, Matheu MP, Sun F, et al. Low-energy Shock Wave Therapy Ameliorates Erectile Dysfunction in a Pelvic Neurovascular Injuries Rat Model. The journal of sexual medicine. 2016;13:22–32. [DOI] [PubMed] [Google Scholar]
- 16.Lin G, Reed-Maldonado AB, Wang B, et al. In Situ Activation of Penile Progenitor Cells With Low-Intensity Extracorporeal Shockwave Therapy. The journal of sexual medicine. 2017;14:493–501. [DOI] [PubMed] [Google Scholar]
- 17.Wu AK, Zhang X, Wang J, et al. Treatment of stress urinary incontinence with low-intensity extracorporeal shock wave therapy in a vaginal balloon dilation induced rat model. Translational andrology and urology. 2018;7:S7–S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin G, Alwaal A, Zhang X, et al. Presence of stem/progenitor cells in the rat penis. Stem cells and development. 2015;24:264–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang L, Lin G, Lee YC, et al. Transgenic animal model for studying the mechanism of obesity-associated stress urinary incontinence. BJU international. 2017;119:317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin AS, Carrier S, Morgan DM, Lue TF. Effect of simulated birth trauma on the urinary continence mechanism in the rat. Urology. 1998;52:143–151. [DOI] [PubMed] [Google Scholar]
- 21.Li M, Li G, Lei H, et al. Therapeutic Potential of Adipose-derived Stem Cell-based Microtissues in a Rat Model of Stress Urinary Incontinence. Urology. 2016;97:277 e271–277 e277. [DOI] [PubMed] [Google Scholar]
- 22.Song QX, Balog BM, Lin DL, et al. Combination histamine and serotonin treatment after simulated childbirth injury improves stress urinary incontinence. Neurourology and urodynamics. 2016;35:703–710. [DOI] [PubMed] [Google Scholar]
- 23.Lin G, Ning H, Wang G, Banie L, Lue TF, Lin CS. Effects of birth trauma and estrogen on urethral elastic fibers and elastin expression. Urology. 2010;76:1018 e1018–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Page RC, Benditt EP. Molecular diseases of connective and vascular tissues. II. Amine oxidase inhibition by the lathyrogen, beta-aminopropionitrile. Biochemistry. 1967;6:1142–1148. [DOI] [PubMed] [Google Scholar]
- 25.Herrera-Imbroda B, Lara MF, Izeta A, Sievert KD, Hart ML. Stress urinary incontinence animal models as a tool to study cell-based regenerative therapies targeting the urethral sphincter. Adv Drug Deliv Rev. 2015;82–83:106–116. [DOI] [PubMed] [Google Scholar]
- 26.Lu Z, Lin G, Reed-Maldonado A, Wang C, Lee YC, Lue TF. Low-intensity Extracorporeal Shock Wave Treatment Improves Erectile Function: A Systematic Review and Meta analysis. European urology. 2017;71:223–233. [DOI] [PubMed] [Google Scholar]
- 27.Zissler A, Steinbacher P, Zimmermann R, et al. Extracorporeal Shock Wave Therapy Accelerates Regeneration After Acute Skeletal Muscle Injury. The American journal of sports medicine. 2017;45:676–684. [DOI] [PubMed] [Google Scholar]
- 28.Ashton-Miller JA, Howard D, DeLancey JO. The functional anatomy of the female pelvic floor and stress continence control system. Scand J Urol Nephrol Suppl. 2001:1–7; discussion 106–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee YC, Lin G, Wang G, et al. Impaired contractility of the circular striated urethral sphincter muscle may contribute to stress urinary incontinence in female zucker fatty rats. Neurourology and urodynamics. 2017;36:1503–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang X, Alwaal A, Lin G, et al. Urethral musculature and innervation in the female rat. Neurourology and urodynamics. 2016;35:382–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maharajan N, Vijayakumar K, Jang CH, Cho GW. Caloric restriction maintains stem cells through niche and regulates stem cell aging. J Mol Med (Berl). 2020;98:25–37. [DOI] [PubMed] [Google Scholar]
- 32.Wong W, Crane ED, Kuo Y, Kim A, Crane JD. The exercise cytokine interleukin-15 rescues slow wound healing in aged mice. The Journal of biological chemistry. 2019;294:20024–20038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ogura Y, Sato S, Kurosaka M, Kotani T, Fujiya H, Funabashi T. Age-related decrease in muscle satellite cells is accompanied with diminished expression of early growth response 3 in mice. Mol Biol Rep. 2020;47:977–986. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.