Figure 1. The composition of the 26S proteasome and an illustration of a 26S proteasome nanodomain.
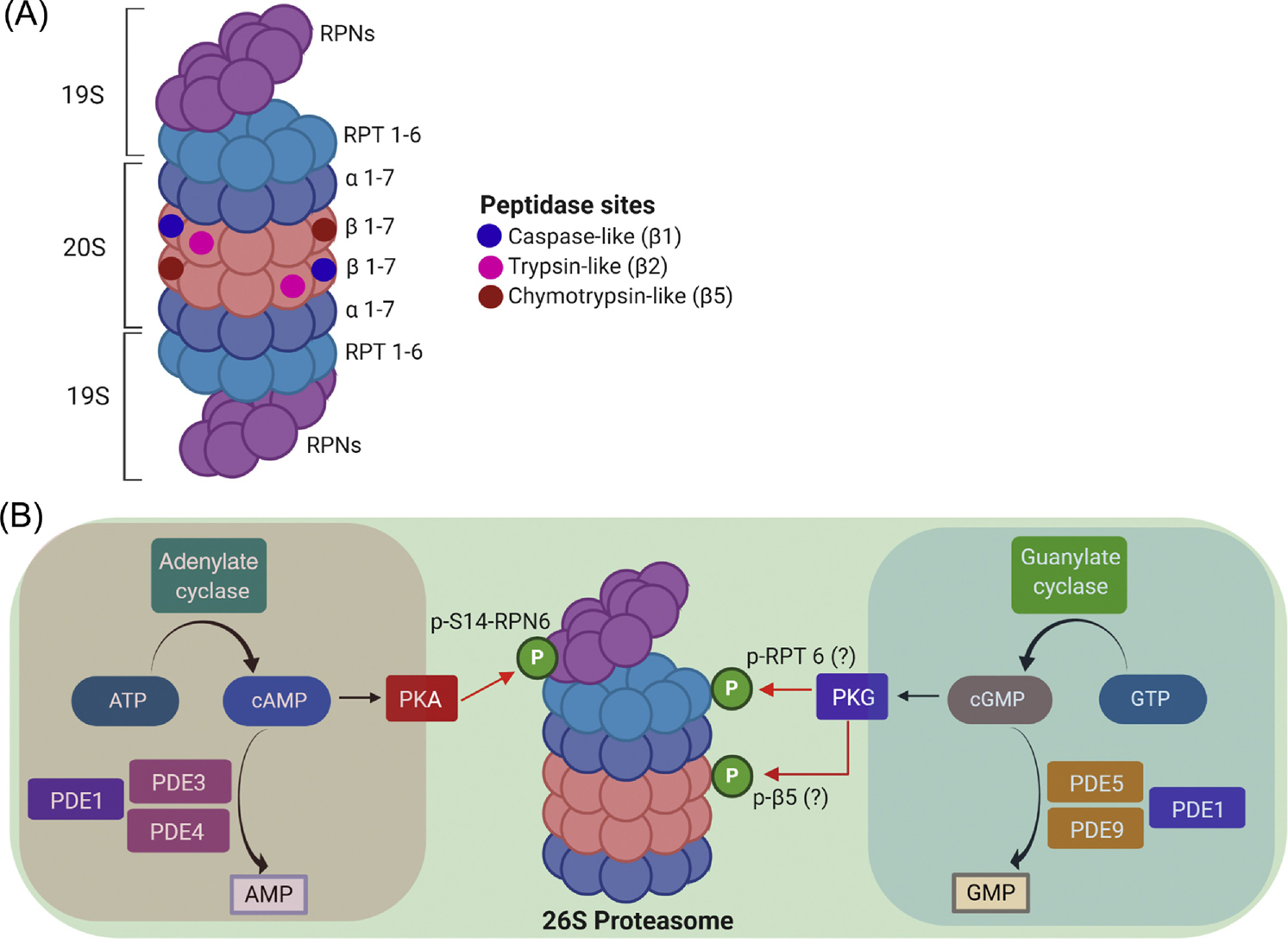
A, The 26S proteasome contains a cylinder-shaped 20S core particle capped by a 19S regulatory particle at one or both ends. The 20S is an axial stack of four rings: two outer α-rings and two inner β-rings. Each ring is formed by 7 protein subunits: α1 through α7 for the α ring and β1 through β7 for the β ring. The proteasomal peptidase activities reside in β1, β2, and β5 subunits while the α ring gates substrate access to the proteolytic chamber of the 20S. The 19S consists of a lid and a base subcomplex; the lid is formed primarily by the non-ATPase subunits RPNs and the base is composed of six ATPase subunits (RPT1 through RPT6). B, An illustration of a 26S proteasome nanodomain. Main regulators of the cAMP/PKA and the cGMP/PKG signaling modules are highlighted in the pink and the blue zone, respectively. Both cAMP/PKA and cGMP/PKG activate the 26S proteasome. PKA does so via the selective phosphorylation of the Ser14 of RPN6 (p-S14-RPN6), a 19S lid subunit strategically positioned to interact with both the base of the 19S and the α-ring of the 20S. PKG primes the proteasome perhaps through phosphorylating RPT6 and β5subunits.
