Figure 2. A schema for proposed duo-activation of PKA and PKG to treat cardiac disease with IPTS.
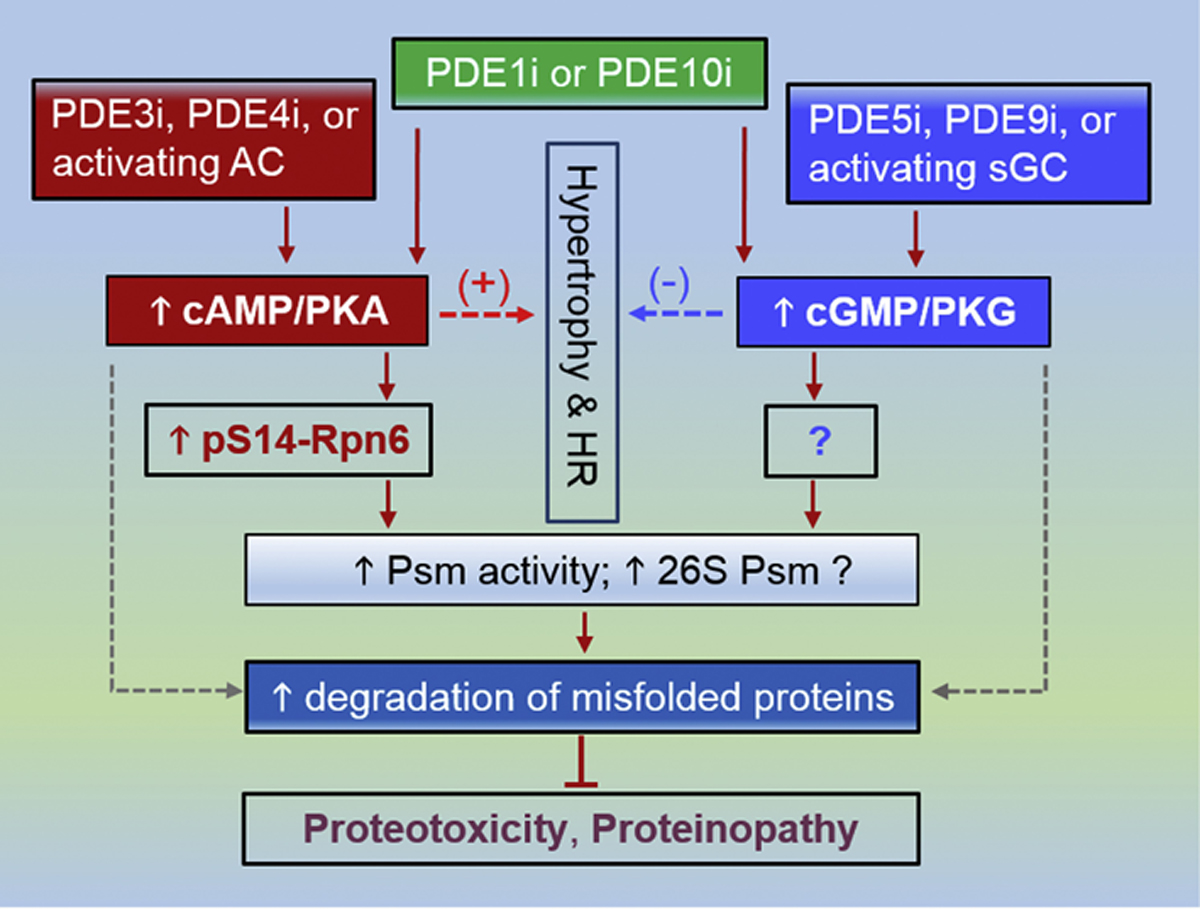
PDE3 inhibition (PDE3i), PDE4 inhibition (PDE4i) as well as activation of the adenylate cyclase (AC) (dark red) are expected to augment cAMP/PKA signaling, which leads to increased Ser14-phosphorylated RPN6/PSMD11 (p-S14-RPN6) and thereby increases proteasome (Psm) activities and perhaps 26S Psm assembly, which in turn facilitates the degradation of misfold proteins and protects proteotoxicity. In parallel, PDE5i, PDE10i or using an activator or stimulator of soluble guanylate cyclase (sGC) (blue) will augment cGMP/PKG signaling, which increases proteasome activities by phosphorylating Psm subunit(s) that remains to be identified and promotes the degradation of misfolded proteins. Stimulating cAMP/PKA promotes cardiac growth (hypertrophy) and increases heart rate (HR), which raises cardiac oxygen consumption and represents undesirable effects. Augmentation of cGMP/PKG signaling, on the other hand, suppresses hypertrophy and decreases HR, which in some cases may not be beneficial either. Hence, duo-activation of PKA and PKG is expected to cancel out some of their undesirable non-Psm effects while complementarily prime the Psm. The duo-activation may be achievable by PDE1 inhibition (PDE1i) or PDE10 inhibition (PDE10i) but PDE1 and PDE10i do not necessarily equally raise cAMP and cGMP in a given cell type; consequently, a proper combination of a method from the PKA activation side and one from the PKG activation side would likely better serve the purpose. The dot lines denote conceivable alternative pathways that currently have no clear support.
