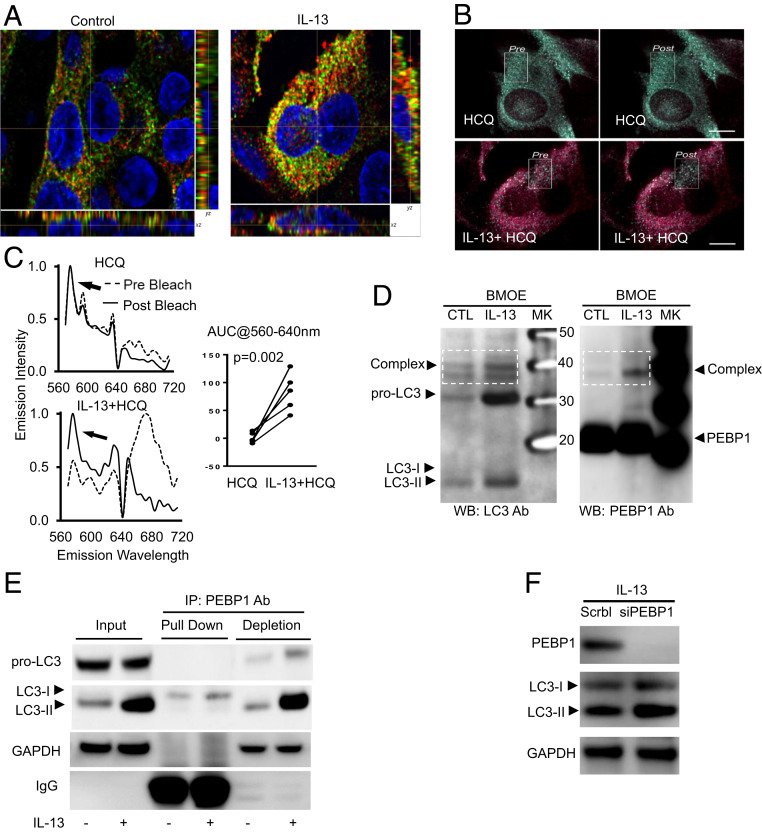
Fig. 2.
Sequestration of LC3 by PEBP1 prevents its lipidation and subsequent activation of autophagy. HAECs were stimulated with/without IL-13 for 7 d, and IF/confocal microscopy and object-based localization were performed. (A) Representative images of object-based colocalization showing IL-13 increased total number of LC3 puncta colocalized with PEBP1 as compared with unstimulated cells. Red, PEBP1; green, LC3; yellow, PEBP1/LC3 colocalization. (B and C) FRET showing IL-13–induced PEBP1–LC3 interactions. HAECs were stimulated with IL-13 for 7 d. Cells were then treated overnight with or without HCQ (10 μM). Images were collected at 60× (14 NA) on a Nikon A1 equipped with a spectral detector. Scale bar, 10 microns. Images are colored based on the wavelength of the peak emissions for each pixel of the image, following the selective excitation of the donor fluorophore (cy3, 540 nm). Green pixels represent the emissions of the donor, and red pixels represent the emissions from the acceptor (cy5) and are indicative of FRET. The absence of red pixels in the untreated samples suggests that minimal FRET occurred, whereas the strong emission from the acceptor in the IL-13–treated samples supports the presence of FRET (and thereby, interaction of PEBP1 and LC3), which was confirmed by acceptor photobleaching within the white boxed region, and the unquenching of the donor emissions (the appearance of green pixels). (D) Cross-linking of total protein with BMOE showing IL-13 increased PEBP1–LC3 complex formation. Total cell lysate protein was incubated with BMOE (0.2 mM) for 2 h at 4 °C, and the reaction was stopped by directly boiling in 2× SDS-DTT sample buffer. Samples were divided and loaded onto two identical SDS/PAGE gels for western blot for LC3 and PEBP1, respectively. (E) Co-IP/WB showing IL-13–induced PEBP1 binding with LC3-I but not with pro-LC3 and LC3-II. (F) siPEBP1 transfection increased IL-13–induced LC3-II protein levels. HAECs transfected with siPEBP1 were all stimulated with IL-13 for 5 d, with scramble siRNA as the negative control. NA, numerical aperture; AUC, area under curve; CTL, control; SDS, sodium dodecyl sulfate; DTT, dithiothreitol; PAGE, polyacrylamide gel electrophoresis; MK, maker; IP, immunoprecipitation; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; WB, western blot.