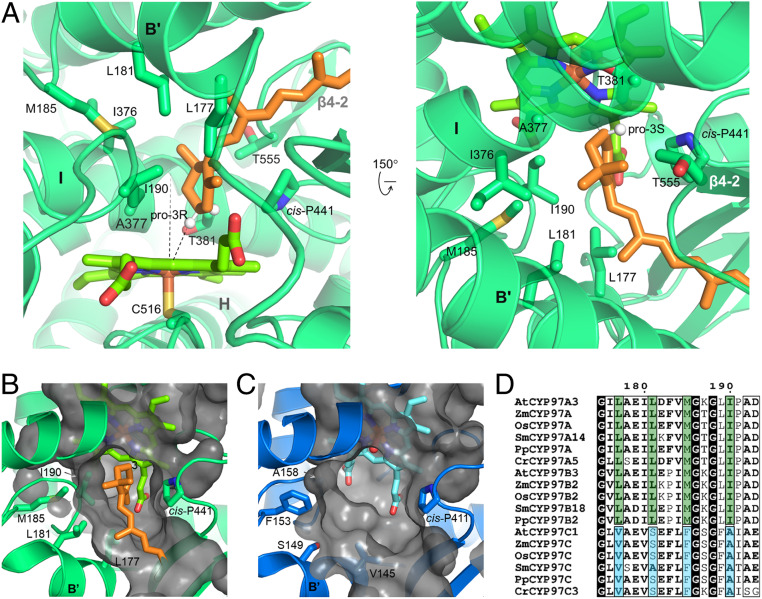
Fig. 5.
Structural basis for pro-3R stereospecificity. (A) The active site in retinal-bound CYP97A3. The protein is shown in transparent ribbons and heme is in bright green. The C-3 hydrogens are positioned and shown as white spheres. The heme normal and the distance between the pro-3R hydrogen and iron are shown as dashes. (B) Surface representation of the CYP97A3 pocket. Side chains of Leu177, Leu181, Met185, and Ile190 are shown. (C) Surface representation of the CYP97C1 pocket. Side chains of Val145, Ser149, Phe153, and Ala158 are shown. (D) Alignment of the B′ helix and B′/C loop. Species include A. thaliana, Z. mays, Oryza sativa, Selaginella moellendorffii, Physcomitrella patens, and Chlamydomonas reinhardtii.