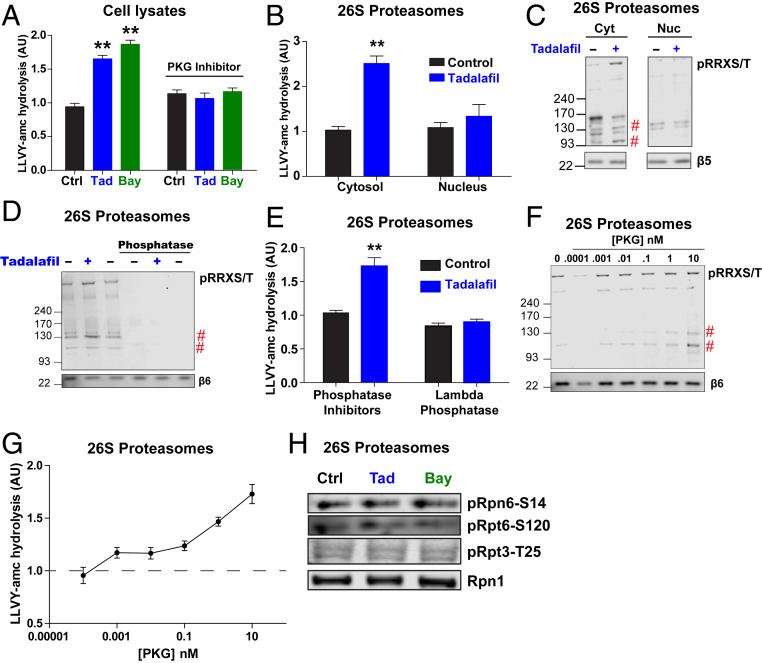
Fig. 2.
PKG directly activates 26S proteasomes by phosphorylation. (A) Inhibition of PKG with Rp-8-pCPT-cGMPs (1 μM) prevented the increase in proteasomal chymotrypsin-like activity caused by 30-min treatment of SH-SY5Y cells with tadalafil or BAY41-2272. n = 4 samples. One-way ANOVA with Dunnett multiple comparison test. **P ≤ 0.01. The experiment was repeated twice with similar results. (B) 26S proteasomes purified from the cytosolic fraction of tadalafil-treated SH-SY5Y cells showed increased chymotrypsin-like activity, but no increase was seen in the activity of 26S proteasomes from the nuclear fraction. n = 3 proteasome purifications. Student’s t test. **P ≤ 0.01. (C) Proteasomes purified from the cytosolic fraction of cells treated for 30 min with tadalafil contained increased amounts of phosphorylated proteins. 26S proteasome preparations from B were analyzed by western blot for phosphorylated RRXSer/Thr. The indicated bands (#) were reproducibly more intense in experiments from all proteasome preparation. Representative western blots from one of three proteasome purifications are shown. No phosphorylated RRXS/T bands were detected below 93 kDa. (D) Incubation of 26S proteasomes purified from SH-SY5Y cells with λ phosphatase for 1 h eliminated all bands containing phosphorylated Ser/Thr residues assayed as in B. Representative western blots from one of three experiments are shown. (E) Dephosphorylation of 26S proteasomes by λ phosphatase (shown in D) reversed the increase in chymotrypsin-like activity induced by treatment of SH-SY5Y cells with tadalafil for 30 min. n = 3 proteasome purifications. Student’s t test. **P ≤ 0.01. (F) Incubation of 26S proteasomes (5 nM) purified from human BJ5A fibroblast cells with increasing concentrations of PKG1α for 1 h caused an increase in the phosphorylation of the indicated bands (#). Representative western blots from one of two experiments is shown. (G) After proteasomes were incubated with increasing concentrations of PKG (F), their chymotrypsin-like activity increased in a progressive manner. The average activity ± SEM of three reactions per time point is shown. (H) Agents that raise cGMP do not cause the phosphorylation of the three sites on the proteasome previously reported to stimulate activity: Rpn6-S14 (13, 15), Rpt3-Thr25 (11), and Rpt6-S120 (27). 26S proteasomes purified from SH-SY5Y cells treated for 30 min with tadalafil or BAY41-2272 were analyzed by western blot for antibodies specific for the indicated phosphorylated subunits. Representative western blots from one of four proteasome purifications are shown.