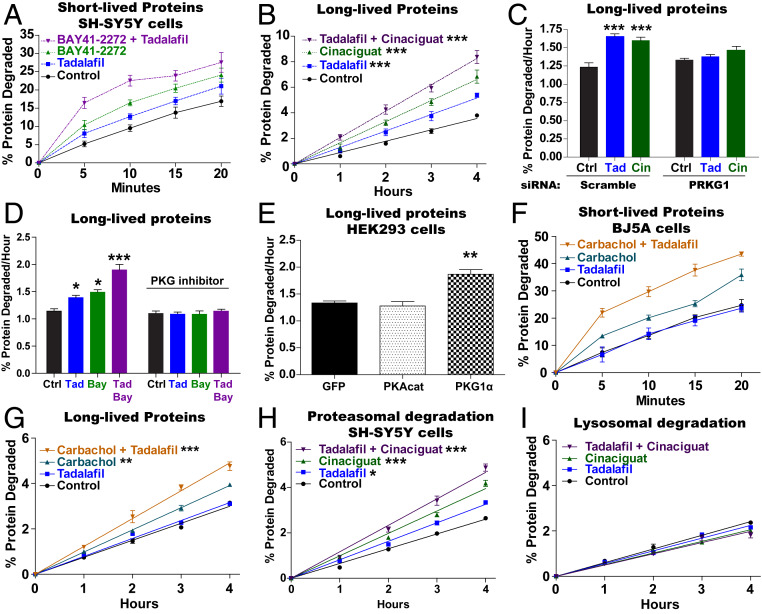
Fig. 3.
Raising cGMP stimulates total intracellular protein breakdown by the UPS. (A) Raising cGMP stimulates the degradation of short-lived intracellular proteins. SH-SY5Y cells were incubated for 20 min with 3H-phenylalanine, and then protein degradation was measured in the presence of cycloheximide (150 µg/mL) by following the conversion of 3H-labeled proteins to amino acids in the media. The pharmacological agents were added to the media after the labeling of cell proteins. n = 4. Error bars represent the means ± SEM. (B) The degradation of long-lived proteins, the bulk of cell proteins, was increased by agents that raise cGMP. SH-SY5Y cells were exposed to 3H-phenylalanine for 16 h and then washed and resuspended in chase medium containing excess nonlabeled phenylalanine for 2 h. The conversion of radiolabeled proteins to labeled amino acids in the media was measured. The pharmacological agents were added to the media after the 2-h chase period. Data shown are the slopes calculated from linear degradation rates. n = 3. One-way ANOVA with Dunnett multiple comparison test. ***P ≤ 0.001. (C) Knockdown of PKG with siRNA blocked the cGMP-mediated increase in the degradation of long-lived proteins. SH-SY5Y cells were exposed to scramble- or PRKG1-siRNA for 48 h, and then degradation of long-lived proteins was measured as in B. A representative western blot of one of three knockdowns of PKG is shown in SI Appendix, Fig. S4C. n = 3. One-way ANOVA with Dunnett multiple comparison test. ***P ≤ 0.001. (D) Inhibition of PKG with Rp-8-pCPT-cGMPs (1 μM) blocked the increase in the degradation of long-lived proteins caused by agents that raise cGMP. Protein degradation was measured as in B. n = 3. One-way ANOVA with Dunnett multiple comparison test. *P ≤ 0.05, ***P ≤ 0.001. (E) Overexpression of PKG, but not PKA catalytic subunit, stimulated the degradation of long-lived proteins. HEK293 cells were transfected with cDNA encoding GFP, PKA catalytic subunit, or PKG 6 h before exposure to 3H-phenylalanine. Protein degradation was measured as in B. n = 3. One-way ANOVA with Dunnett multiple comparison test. **P ≤ 0.01. (F) The muscarinic agonist carbachol (1 µM) increases the degradation of short-lived proteins in human fibroblasts (BJ5A) and increases degradation even further in the presence of the PDE5 inhibitor tadalafil. Protein degradation was measured as in A, and all agents were added after the labeling of cell proteins with 3H-phenylalanine. n = 4. (G) Carbachol also increases the degradation of long-lived proteins in human fibroblasts (BJ5A) and increases degradation even further in the presence of the PDE5 inhibitor tadalafil. Protein degradation was measured as in B. n = 3. One-way ANOVA with Dunnett multiple comparison test. **P ≤ 0.01, ***P ≤ 0.001. (H) Degradation of long-lived cell proteins by the proteasome was increased by agents that raise cGMP. Protein degradation was measured as in B, but the contribution of the proteasome was measured by including in the media the inhibitor of lysosomal acidification concanamycin A (200 nM) 1 h after protein labeling. n = 4. One-way ANOVA with Dunnett multiple comparison test. *P ≤ 0.05, ***P ≤ 0.001. (I) Lysosomal protein degradation (autophagy) was not increased by treatments that raise cGMP. The degradation of long-lived proteins was measured as in B, and to measure the contribution of the lysosome, the proteasome inhibitor carfilzomib (1 µM) was added to the media in the second hour of the chase period and was present throughout the measurements of degradation. n = 4.