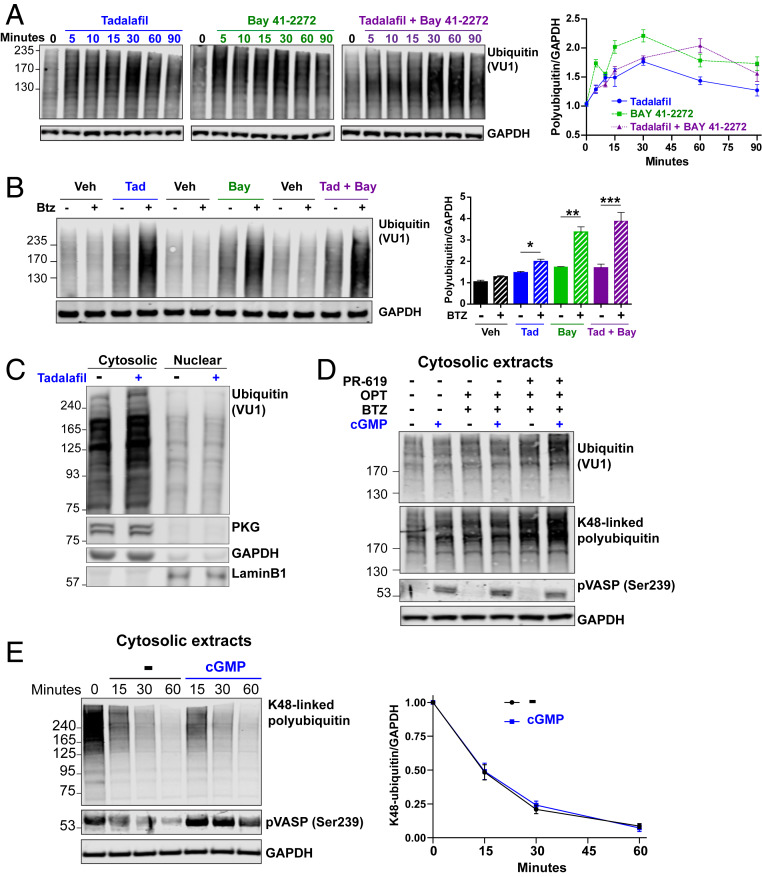
Fig. 4.
cGMP also rapidly increases levels of ubiquitin conjugates by stimulating ubiquitylation of cell proteins. (A) SH-SY5Y cells were treated with agents that raise cGMP and the cell lysates were analyzed by western blot for ubiquitin. The levels of polyubiquitinated proteins were quantified and representative western blots from one of three experiments are shown. n = 3. Averages ± SEM are shown. (B) The increase in ubiquitin conjugates caused by agents that raise cGMP is even greater in the presence of the proteasome inhibitor bortezomib. SH-SY5Y cells were treated for 15 min with these agents, lysed, and analyzed by western blot for ubiquitin, as in A. Representative western blots of one of four experiments are shown. n = 4. One-way ANOVA with Bonferroni’s multiple comparison test. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001. (C) Raising cGMP with tadalafil for 30 min in SH-SY5Y cells increases levels of polyubiquitinated proteins in the cytosolic fraction, but not the nuclear fraction. Representative western blots of one of three experiments are shown. (D) cGMP increases ubiquitination of cytosolic proteins in a cell-free system. HEK293 cells were lysed in a hypotonic buffer, and then cytosolic extracts were prepared by pelleting the nucleus (800 × g for 15 min) and mitochondria (10,000 × g for 10 min). The extracts were incubated for 30 min at 37 °C with or without cGMP (1 µM) in the presence of bortezomib (1 µM), 1,10-phenanthroline (250 µM), and PR-619 (10 µM) to prevent deubiquitination and proteasomal degradation of ubiquitin conjugates. Representative western blots of one of four experiments is shown. (E) cGMP does not change the rate of deubiquitination of cytosolic proteins in a cell-free system. HEK293 cells were treated for 30 min with bortezomib and then cytosolic extracts were prepared as in D, except an inhibitor of the ubiquitin activating enzyme (TAK243, 1 µM) was included instead of inhibitors of deubiquitinases. Representative western blot is shown of one of three experiments. n = 3.