Fig. 5.
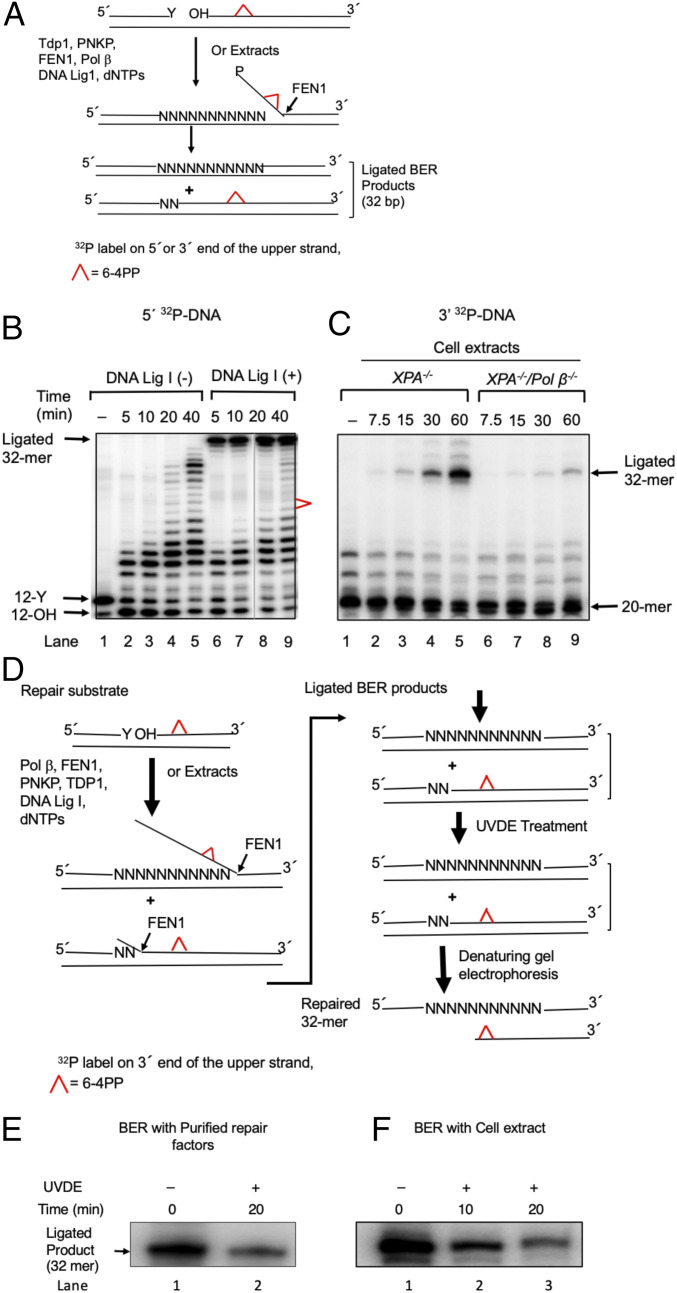
In vitro long-patch BER synthesis removes the 6–4PP UV lesion. (A) Schematic representation of in vitro BER of a 6–4PP. Top represents the repair substrate. A 32-bp duplex DNA with a nick was prepared by annealing 32-mer template with both a 12-mer upstream primer having 3′ Y (tyrosine) and a 20-mer downstream sequence containing a 6–4PP lesion at positions 9 and 10. The tyrosine residue at the nick mimics a postproteasomal removal of stable TOP1ccs. The 5′ end of the 20-mer had a hydroxy residue. TDP1 removes the tyrosine residue, and polynucleotide kinase phosphatase (PNKP) cleaves the 3′-phosphate group and also phosphorylates the 5′ end of the 20-mer. POLβ inserts nucleotides (N) creating flap structures that are incised by FEN1, and DNA ligase I seals the resulting nick, leading to the formation of ligated BER products. The upper strand was labeled either at the 5′ end by 32P[γATP] (B) or at the 3′ end by 32P[αddCTP] (C). (B) Repair of 6–4PP lesion-containing DNA substrate by purified BER factors in vitro. The BER reaction was conducted without DNA ligase I (−) (lanes 2–5) or with DNA ligase I (lanes 5–9), and reaction products were analyzed at the indicated times. The positions of 32P-labeled substrate (12-Y), the products of TDP1 and PNKP catalysis (12-OH), the ligated 32-mer product, and 6–4PP are indicated. The results shown are typical of three experiments. (C) Repair of 6–4PP lesion-containing DNA by cell extracts. The BER reaction was conducted either with an XPA−/− extract (lanes 2–5) or with an XPA−/−/POLβ −/− extract (lanes 5–9), and the reaction products were analyzed at the indicated time intervals. The results show a requirement of POLβ to repair the 6–4PP lesion in cell extracts. The positions of the substrate (20-mer), BER intermediates, and ligated 32-mer products are indicated. The results shown are typical of three experiments. (D) Schematic representation of the method of analyzing the removal of a 6–4PP from the repair substrate and ligated 32-mer products. BER reaction mixtures were treated with UVDE that selectively incises 5′ to the 6–4PP lesion. Therefore, if the 6–4PP lesion was not repaired, the ligated product (32 bp) would be sensitive to UVDE digestion. However, if the 6–4PP lesion was repaired, the ligated product would be insensitive to UVDE digestion, as illustrated. (E and F) Repair of the 6–4PP lesion in in vitro BER. The repair reaction was performed with purified enzymes as in B (lane 9) or with an XPA−/− cell extract as in C (lane 5), respectively. Analysis of the ligated products was shown. Reaction mixtures were subjected to a UVDE treatment. The repaired products after 20-min (E) or 10- and 20-min (F) incubations with UVDE were analyzed. The ‘0’ time represents untreated reaction mixture. UVDE-resistant ligated products indicate repair of the 6–4PP lesion. Results show a significant amount of the 6–4PP lesion repair in both BER systems, the reconstitution with purified BER factors (E) and extract-based BER (F), respectively. A representative phosphorimage of two experiments is shown.