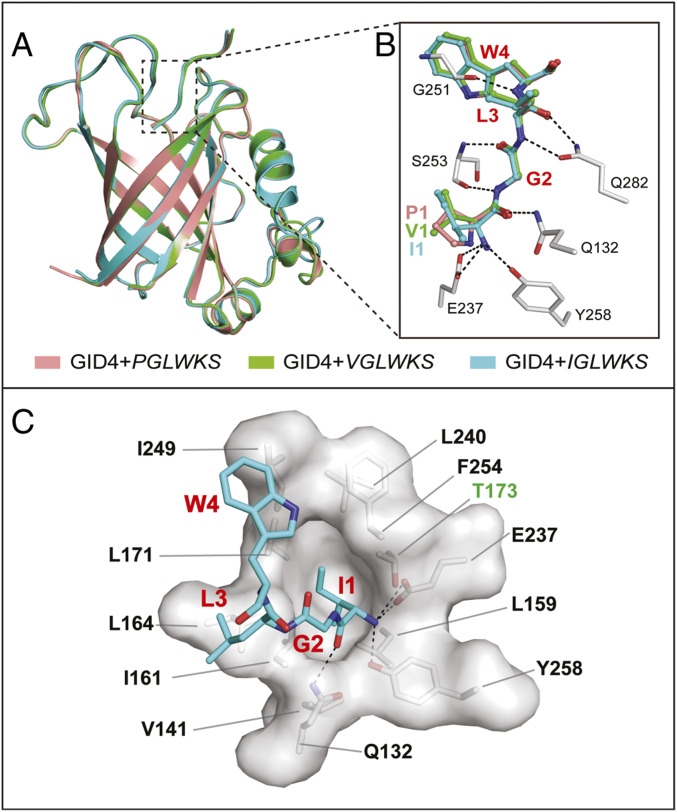
Fig. 4.
Structural basis of recognition of nonproline N-terminal residues by human GID4. (A) Superposition, in ribbon diagrams, of the overall crystal structures of complexes of GID4124–289 with, respectively, PGLWKS (salmon, PDB ID code 6CDG), VGLWKS (green), and IGLWKS (cyan) peptides (see also Materials and Methods and SI Appendix, Table S1). (B) Close-up view of similar (conserved) interactions between the substrate-binding chamber of GID4 and the first four residues of the above peptides (PGLW, salmon; VGLW, green; and IGLW, cyan). The most relevant residues of GID4 are shown as gray/red/blue sticks vis-à-vis colored peptides. Hydrogen bonds are indicated as black dash lines. (C) Close-up view of the recognition of the Nt-Ile–bearing IGLWKS peptide in the substrate-binding chamber of human GID4. Denoted in red are the first four residues of the six-residue peptide. These residues are depicted as cyan/red/blue sticks. The most relevant residues of the GID4 chamber are either in black or in green (the hydrophilic Thr-173 residue), and are depicted via surface representations and gray sticks.