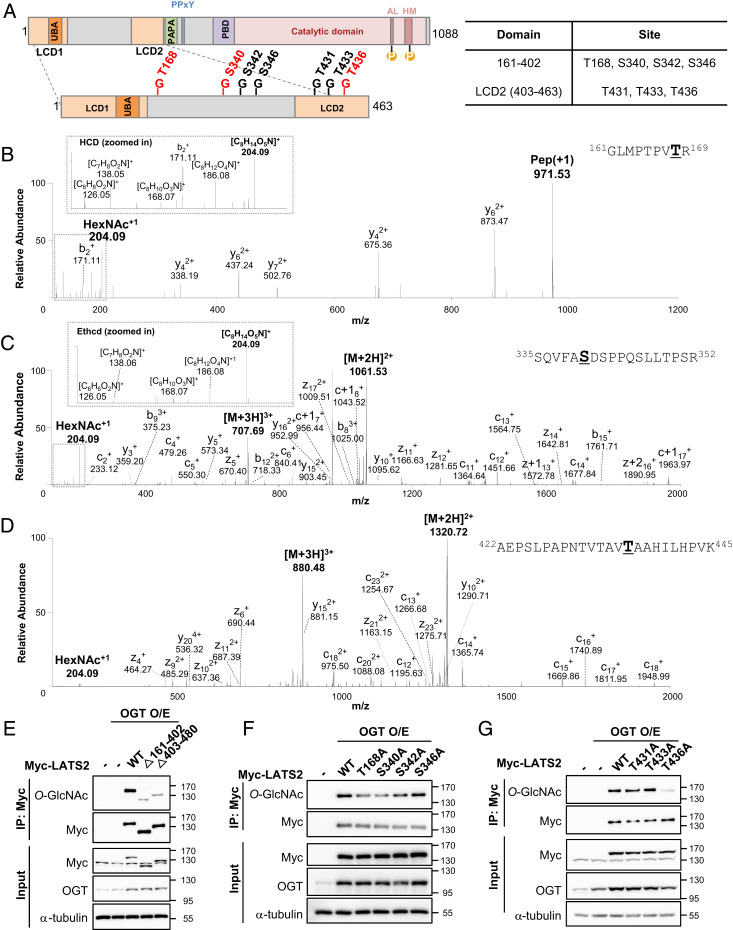
Fig. 3.
LATS2 O-GlcNAcylation sites were identified. (A) A schematic drawing of the LATS2 O-GlcNAcylation regions based on mass spectrometry data (Fig. 3 B–D and SI Appendix, Fig. S2). Abbreviations: LCD1 (LATS conserved domain 1), LCD2 (LATS conserved domain 2), PDB (protein binding domain), UBA (ubiquitin-associated domain), AL (activation loop), HM (hydrophobic motif), PAPA (sequence with repeats of proline-alanine residues), PPxY (proline-proline-any amino acid-tyrosine). Red letters indicate major LATS2 O-GlcNAcylation sites. (B–D) O-GlcNAcylation was detected at Thr168 (B), Ser340 (C), and Thr436 (D). (B) This peptide corresponds to residues 161 to 169, GLMPTPVTR. The O-GlcNAc oxonium ion (m/z, 204.09) and a series of its fragments (m/z, 186.08, 168.07, 138.05, and 126.05) were also assigned. (C) This peptide corresponds to residues 335 to 352, SQVFASDSPPQSLLTPSR. The O-GlcNAc oxonium ion (m/z, 204.09) and a series of its fragments (m/z, 186.08, 168.07, 138.06, 126.05) were also assigned. (D) This peptide corresponds to residues 422 to 445, AEPSLPAPNTVTAVTAAHILHPVK. The O-GlcNAc oxonium ion (m/z, 204.09) was also assigned. (B–D) O-GlcNAcylation detected residues are bolded and underlined. (E–G) HEK293 cells were cotransfected with each indicated Myc-LATS2 and OGT. (E) Each immunoprecipitated LATS2 wild-type and deletion mutants was immunoblotted with anti-O-GlcNAc antibody. Abbreviations: Δ161-402 (deletion of the linker region between LCD1 and LCD2), Δ403-480 (deletion of LCD2 and a PAPA repeat). (F and G) Each overexpressed Myc-LATS2 in HEK293 cells was immunoprecipitated with Myc antibody-conjugated A/G agarose beads. Western blotting was performed with anti-O-GlcNAc antibody.