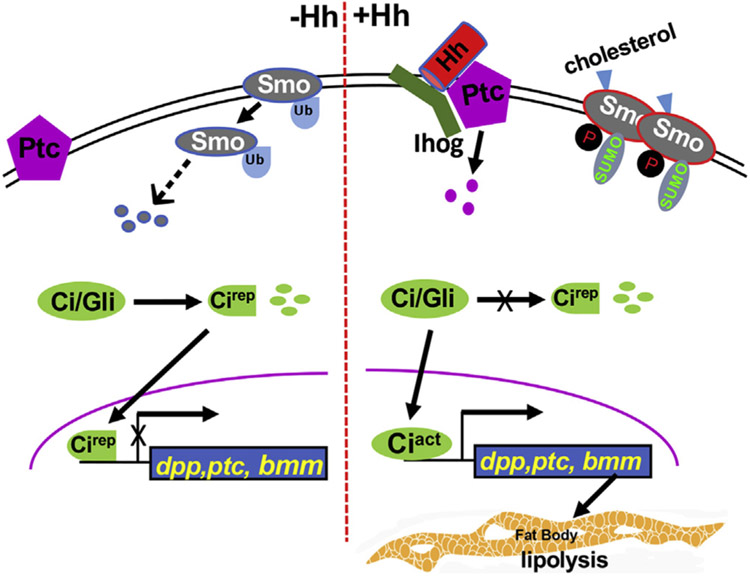
Fig. 7. A model of Hh signaling and its connection with lipolysis.
In the absence of Hh, Ptc inhibits Smo. Smo undergoes endocytosis that is mediated by ubiquitination. Ci/Gli is processed into a truncated repressor form that enters into the nucleus to block the expression of target genes, such as dpp and ptc. In the presence of Hh, the Ptc inhibition on Smo is relieved, causing increased Smo phosphorylation and accumulation on the cell surface. Phosphorylation and sumoylation counteracts ubiquitination to activate Smo. Upon Hh stimulation, full-length Ci is activated to turn on target gene expression. In this study, Bmm is identified as a direct target of Hh signaling. Hh signaling promotes lipolysis through Ci binding to the bmm promoter, therefore, elevates Bmm transcription.