Figure 1. Approved monoclonal antibodies and bNAbs tested in clinical trials.
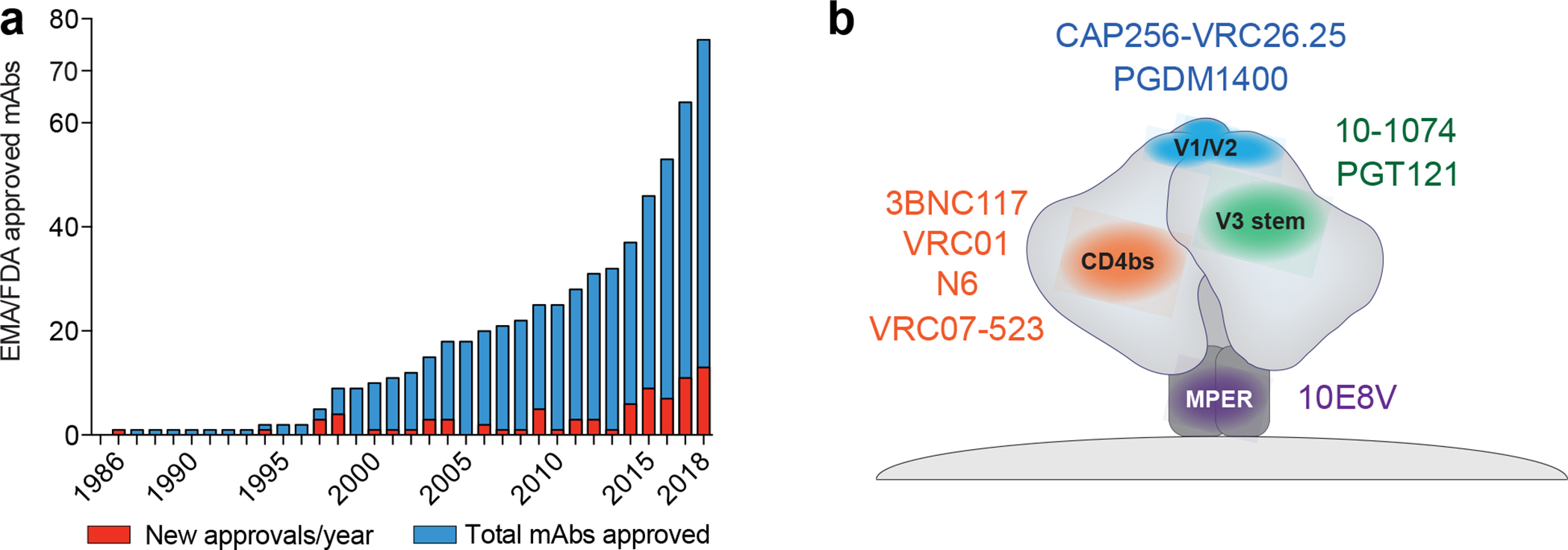
a.) Number of EMA (European Medicines Agency) and FDA (U.S. Food and Drug Administration) approved monoclonal antibodies up until the end of 2018. Red columns indicate the number of newly approved mAbs in a given year. Blue columns show the number of total mAbs approved2. b.) Second generation bNAbs target various epitopes on the HIV-1 envelope trimer (grey). Antibodies against the CD4bs (orange; 3BNC117, VRC01, VRC07–523, N6), the V1/V2 loop (blue; PDGM1400), the V3-stem (green; 10–1074, PGT121), and the Membrane Proximal External Region (MPER; purple; 10E8V) are currently being investigated in clinical studies.
