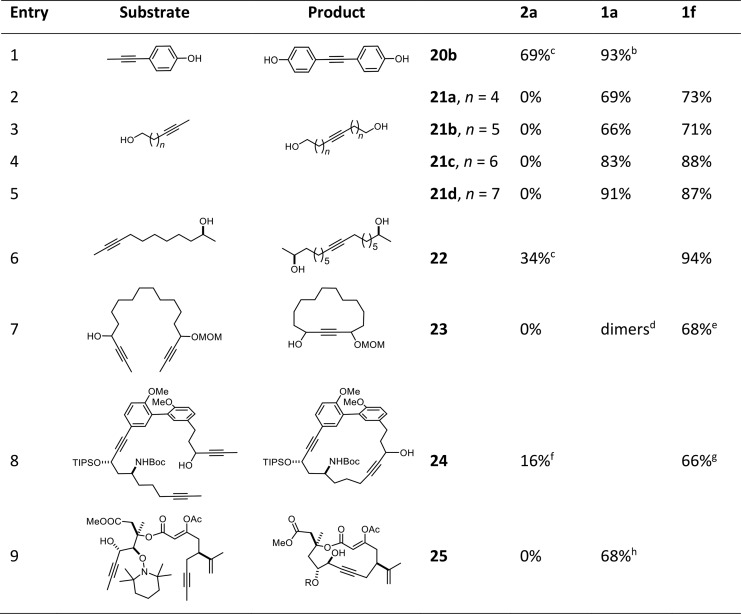
Table 2. Homo-Metathesis and Ring-Closing Alkyne Metathesis Reactions in the Presence of Unprotected −OH Groupsa.
Isolated yields of reactions performed with 5 mol % of catalyst loading in toluene at RT in the presence of MS 5 Å, unless stated otherwise.
At 60 °C.
With 10 mol % of 2a.
Mostly formation of dimers (NMR).
With 10 mol % of catalyst at 110 °C.
Yield determined by high-performance liquid chromatography (HPLC); in addition, ca. 20% of what appeared to be dimeric products were detected.
With 20 mol % of catalyst at 80 °C.
Using 30 mol % of catalyst at reflux temperature; yield over two steps (metathetic ring closure and reductive cleavage of the 2,2,6,6-tetramethylpiperidinyl group).