Abstract
The development of new therapeutics is critically dependent on an understanding of the molecular pathways whose disruption results in neurological symptoms. Genetic and biomarker studies have highlighted immune signalling as a pathway that is impaired in patients with neurodevelopmental disorders (NDDs), and several studies in animal models of aberrant neurodevelopment have implicated microglia, the brain’s immune cells, in the pathology of these diseases. Despite the increasing awareness of the role of immune responses and inflammation in the pathophysiology of NDDs, the testing of new drugs rarely considers their effects in microglia. In this brief review we present evidence of how the study of microglia can be critical for understanding the mechanisms of action of candidate drugs for NDDs and for increasing their therapeutic effect.
Microglia are well known for their role as the brain’s immune cells, but recent research has highlighted their significant role in physiological processes during normal brain development. This role could be highly relevant to microglial contributions to NDDs (Fig. 1). During the postnatal development of the uninjured mouse brain, microglia engulf synaptic material and play a major role in synaptic pruning [1]. The pruning process may be affected by experience, as altered sensory input affects various modalities of microglial interaction with synapses. One study found that with light deprivation and re-exposure, microglial processes change morphology, display phagocytic structures, appose synaptic clefts more frequently, and envelope synapse-associated elements more extensively. Light deprivation causes microglia to become less motile and change their localisation to a subset of dendritic spines. This study suggests that microglia may contribute to experience-dependent modification of specific subsets of synapses [2]. This hypothesis is supported by another study which focus on purinergic receptor P2Y12. P2Y12 is selectively expressed in non-inflammatory microglia. Disruption of this receptor alters microglial response to monocular deprivation in mice, again suggesting that microglia actively partake in experience-dependent plasticity [3]. Microglial participation in sensory and activity-driven synapse pruning is a common phenomenon across the developing brain, including in the thalamus [4], somatosensory cortex (PMID: 31209379) and cerebellum (PMID: 30026565). In this context, microglia are capable of sensing and responding to induction of synaptic plasticity, as is seen in the hippocampus where after induction of long-term potentiation, microglia increase their number of processes, and increase their duration of contact with dendritic spines [5].
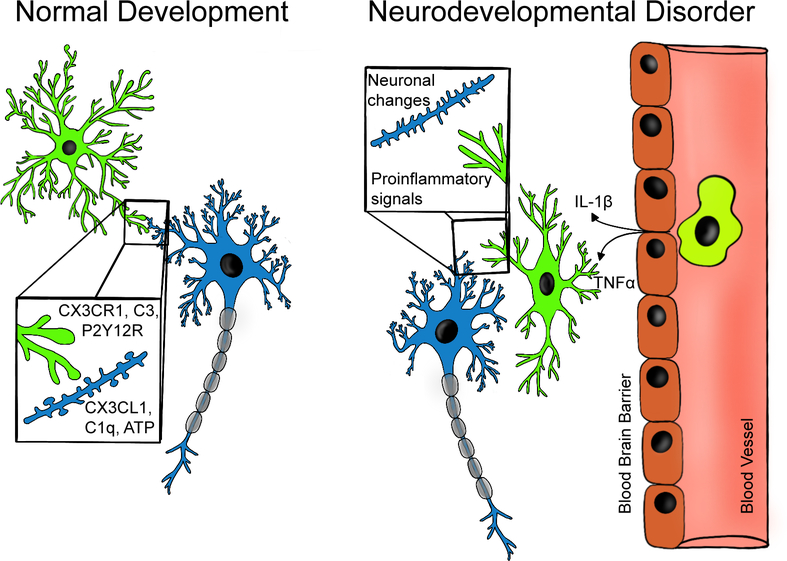
FIGURE 1 |.
Microglial are active participants in neuronal circuit development and maintenance. Left panel: microglia participate in synaptic plasticity and pruning through numerous signaling mechanisms related to immune function including: complement system driven phagocytosis of synapses, CX3CR1 mediated regulation of circuit development, P2Y12 receptor (P2Y12R) dependent synaptic plasticity. Right panel: Changes in microglial function in NDDs may interfere with neuron-microglia signaling leading to aberrant development of neuronal circuits. Interactions between microglia and peripheral immune signaling may potentiate neuroinflammation. These neuroinflammatory loops may be potent therapeutic targets for modulating NDD symptoms.
This role in development is thought to be related to the immune function of microglia. The process of microglial-mediated synaptic pruning has been shown to be dependent on the complement system. In the postnatal retinogeniculate system, microglia engulf presynaptic inputs. This process is dependent on neuronal activity and on the microglia-specific phagocytic pathway, which uses complement receptor 3 (CR3). Disruption of CR3 signalling causes deficits in structural remodelling, leading to increased synaptic density due to impaired pruning by microglia [4]. In the somatosensory cortex, microglial-mediated synaptic pruning is also dependent on the fractalkine system (PMID: 31209379), which allows microglia-neuron communication and has largely been studied in the context of inflammatory signalling [6].
The importance of various microglial functions in normal development suggests that microglia may be an important therapeutic target for NDDs (Fig. 1). Furthermore, a growing body of research posits that inflammation plays an important role in the pathophysiology of NDDs. Xu et al. (2015) reviewed cytokine expression in various tissues of patients with autism spectrum disorder (ASD), and found that the data showed a possible relationship between cytokine alterations and autism, although they cautioned that there were inconsistencies in some results [7]. Notable results included increased Tumour Necrosis Factor (TNF)-α in the peripheral blood mononuclear cells, cerebrospinal fluid and brains of patients with ASD, and increased Interleukin (IL)-1β secreted from the peripheral blood mononuclear cells of children with ASD. These inflammatory cytokines could signal inflammatory changes in the brain that could greatly impact neurodevelopment and neural network function (Fig. 1). Given that microglia are the brain’s immune cells and could be participating in or even causing cytokine elevations, more research is required as to their reliability as a therapeutic target in autism.
In fact, evidence exists directly linking changes in microglia to ASD pathophysiology. Marked microglial activation, leading to neuroinflammation, has been seen in brain tissues of ASD patients compared to neurotypical controls [8–10]. In a post-mortem study, the density of ramified (resting state) microglia was significantly lower in both the grey and white matter of ASD patients compared to typically developing controls [11]. The study also found that around age 2–3 years, ASD patients showed greater numbers of rod and reactive microglia than typically developing patients. From ages 8–32, typically developing patients showed more ramified microglia than any other form, whereas ASD patients showed more primed microglia than ramified. The study also observed clusters of microglia in both grey and white matter. These were seen more frequently in ASD patients than controls. Differences in the microglia of ASD patients have also been detected on a transcriptional level. Single-nucleus RNA sequencing has shown that microglia from patients with ASD showed increased activity of genes associated with microglial activation and transcriptional factors that regulate developmental processes [12]. Changes in microglia, in addition to Layer 2/3 cortical neurons, were found to be most predictive of clinical severity.
A growing body of research suggests that targeting microglial activation is beneficial in ASD. Numerous anti-inflammatory therapies show promise, with proposed pharmacological mechanisms including inhibition of microglial activation and restoration of synaptic function [13]. These therapies include oxytocin, which has been shown to reduce numbers of activated microglia and reduce neuroinflammation, as well as improve the behaviour of mice that model ASD [14]. Additionally, sulforaphen, a compound obtained from cruciferous vegetables, resveratrol, found in grapes, and vitamin-D are all an anti-oxidants and anti-inflammatory agents that could also decrease neuroinflammation caused by reactive microglia in the context of ASD [13].
Microglial abnormalities have also been implicated in Rett Syndrome (RTT). RTT is a severe neurodevelopmental disorder often caused by mutations in the Methyl-CpG-binding protein 2 (MECP2) [15]. MECP2 has also been found to be aberrantly expressed in ASD [16]. Although numerous studies have been carried out on Mecp2-null microglia, the extent and role of microglial involvement remains unclear. We will discuss a number of proposed mechanisms and important studies.
Early studies in culture, suggested that Mecp2-null microglia are neurotoxic as they release five times more glutamate at baseline in vitro. This increased glutamate is toxic to cultured hippocampal neurons, suggesting that microglia contribute to the pathophysiology of RTT [17]. In 2012, it was reported that the transplantation of wild-type microglia into Mecp2-null mice prevented neurological decline and early death [18]. However, these findings were not replicated two years later [19], and over a time a more tempered view of microglial function in RTT has evolved. Currently, it appears clear that microglia are altered in RTT but their pathology is likely related to primary changes in neurons or other cell types.
In the RTT mouse model, differences are seen in microglia before any symptom onset occurs in the animal. In a transcriptomic analysis, the microglia of control female mice and heterozygous female mice carrying one Mecp2-null allele showed differential expression of genes related to innate immunity [20]. This differential expression was observed both prior to onset of neurological symptoms, at 5 weeks, and after their onset, at 24 weeks, although different sets of genes were differentially expressed at each time point. A number of heat shock proteins were differentially expressed at 5 weeks but not 24 weeks, with the authors suggesting pre-phenotypic mice may have alterations in their capacity to respond to heat stress and other stressors.
In an in vivo study, the microglia of late phenotypic Mecp2 null mice have been shown to be larger in size than wild-type [21]. Furthermore, while pre-phenotypic microglia were similar to wild-type, late phenotypic microglia had significantly reduced process complexity in the hippocampus, neocortex, and cerebellum. Progressive loss of microglia was seen from pre- to late-phenotypic stages. The authors posited that these findings together suggest that during disease progression, microglia become activated and are lost. The study also found that Mecp2 has a role in the regulation of microglial hypoxia-induced responses.
However, a recent in vivo study suggested that the role of microglia is mainly limited to the end stage of RTT [22]. It found that in late phenotypic Mecp2-null mice, microglia excessively engulf presynaptic inputs. Interestingly, microglia-specific loss of Mecp2 did not produce the same result, but reintroducing the Mecp2 gene solely into the microglia of the otherwise Mecp2 null mice did still result in excessive engulfment. When Mecp2 was specifically expressed in microglia, there was no significant improvement in neurological score, weight loss, or visual acuity, and only a small improvement in rotarod performance. The authors suggest these findings demonstrate that microglia respond secondarily and engulf synapses in response to circuits rendered vulnerable by Mecp2 loss in other CNS cells.
Numerous hypotheses regarding the role of microglia in RTT relate to their interaction with neurons. Microglial processes regularly make contact with synapses, and microglia regulate neuronal activity [13]. Horiuchi et al. blocked neuron-microglia interaction by ablation of fractalkine signalling, through deletion of chemokine (C-X3-C motif) receptor 1 (CX3CR1) in Mecp2 knockout mice [23]. CX3CR1 is a chemokine receptor expressed in microglia that pairs with its neuronal ligand CX3CL1, facilitating neuron-microglia interaction. CX3CR1 ablation prolonged the lifespan of Mecp2 knockout mice, and improved their weight gain, symptomatic scores, major respiratory parameters, and motor co-ordination and performance. The authors suggested that blocking neuron-microglia interaction prevents aberrant suppression of neuronal activity caused by disruptions in glutamate, as well as decreasing the synaptotoxicity caused by high glutamate levels. Also, the study found that microglia produced higher amounts of insulin-like growth factor 1 (IGF1) after CX3CR1 ablation, comparable to previous IGF1 replacement therapies. Targeting CX3CR1 pharmacologically or genetically was posited as a novel therapeutic target for RTT.
In another therapeutic attempt aimed at microglia, Nance et al. used dendrimer-conjugated N-acetyl cysteine (D-NAC) to target microglia in Mecp2-null mice [24]. N-acetyl cysteine (NAC) is commonly used clinically, with an ability to attenuate oxidative stress, neuroinflammation, glutamate dysregulation, and other pathological processes [25]. It has shown limited efficacy treating irritability in children with ASD [26].
Nance et al. performed in vitro studies of primary mixed glial cells, finding that D-NAC is taken up and retained in the activated glia, releasing the drug over time. In vivo, D-NAC was administered intraperitoneally into Mecp2-null mice and wild-type controls. The dendrimers were localised in the microglia of the Mecp2-null mice, but not in aged-matched wild-type controls. To study the effect of D-NAC, D-NAC-treated Mecp2-null mice were compared to NAC-treated Mecp2-null mice and phosphate buffer solution (PBS)-treated Mecp2-null mice. Treatment was started on postnatal day 21, the average age for symptom onset. D-NAC treated mice showed significant behavioural improvement by postnatal day 35. By 7 weeks of age, PBS-treated mice were emaciated, unable to groom, and had their hind paws clenched. D-NAC treated mice maintained their appearance for longer and had less hind paw clenching. However, NAC and D-NAC did not alter weight loss. D-NAC did not improve survival compared to PBS. The 50% survival rate of both D-NAC and PBS treated pups was 49 days. The 50% survival of the NAC-treated mice was much lower at 25 days (approaching significance) [24]. This microglia-targeted approach holds promise as a potential clinically useful therapy for RTT patients, and warrants further study.
An interesting new research direction, which could help to elucidate the mechanisms by which microglia are dysregulated in ASD, has been the microbiome, which has recently been implicated in microglial changes. Microglia in germ free mice show differential expression of mRNA, including for genes associated with cell activation and immune signal transduction, compared to specific pathogen free mice [27]. It has been suggested that the microbiome plays a role in neuroinflammation in NDDs [28]. This is an exciting and very active area of research in both human populations and animal models.
In summary, we have considered the role of microglia in the pathogenesis and progression of NDDs, particularly ASD and RTT. Increased interest in the role of microglia in NDDs stems from the importance of microglia in normal development, as well as the evidence that a neuroinflammatory state is present in NDDs (Fig. 1). While the extent of the role played by microglia in primary damage in NDDs is as yet unclear, their malfunction may cause impairment through numerous mechanisms. However, pharmacological testing of drugs for NDDs often does not consider the microglia. It is unknown whether current therapeutics may act, in part, through microglial pathways. Additionally, further research should be carried out regarding the precise mechanisms of microglial involvement in NDDs in order to identify novel therapeutic targets related to microglia, such as CX3CR1 and D-NAC.
Acknowledgements
This publication has emanated from research supported in part by a research grant from Science Foundation Ireland (SFI) under Grant Number 16/RC/3948 and co-funded under the European Regional Development Fund and by FutureNeuro industry partners. DT is a recipient of the IRSF grant 3507-2017, and of the Meath Foundation Research grant 2019 (coPI).
Abbreviations
- NDD
Neurodevelopmental Disorders
- CR3
Complement Receptor 3
- TNF
Tumour Necrosis Factor
- IL
Interleukin
- ASD
Autism Spectrum Disorder
- RTT
Rett Syndrome
- MeCP2
Methyl-CpG-binding protein 2
- CX3CR1
Chemokine (C-X3-C motif) receptor 1
- IGF1
Insulin-like Growth Factor 1
- D-NAC
Dendrimer-conjugated N-acetyl Cysteine
- NAC
N-acetyl Cysteine
- PBS
Phosphate Buffer Solution
Footnotes
Conflict of interest
Authors declare No conflict of Interest
Consent for publication
All authors consent to the publication
References
- 1 -.Paolicelli R, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Giuducci E, Dumas L, Ragozzino D, Gross CT. Synaptic Pruning by Microglia Is Necessary for Normal Brain Development. Science, 2011, 333(6048), 1456–1458. [DOI] [PubMed] [Google Scholar]
- 2 -.Tremblay M, Lowery R, Majewska A. Microglial Interactions with Synapses Are Modulated by Visual Experience. PLoS Biol, 2010, 8(11), e1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3 -.Sipe G, Lowery R, Tremblay M, Kelly E, Lamantia C, Majewska A. Microglial P2Y12 is necessary for synaptic plasticity in mouse visual cortex. Nature Comms. 2016, 7(1), 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4 -.Schafer D, Lehrman E, Kautzman A, Koyama R, Mardinly A, Yamasaki R, Ransohoff R., Greenberg M, Barres B, Stevens B. Microglia Sculpt Postnatal Neural Circuits in an Activity and Complement-Dependent Manner. Neuron, 2012, 74(4), 691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5 -.Pfeiffer T, Avignone E, Nägerl U. Induction of hippocampal long-term potentiation increases the morphological dynamics of microglial processes and prolongs their contacts with dendritic spines. Scientific Reports. 2016, 6(1), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6 -.Jung S, Aliberti J, Graemmel P, Sunshine M, Kreutzberg G, Sher A et al. Analysis of Fractalkine Receptor CX3CR1 Function by Targeted Deletion and Green Fluorescent Protein Reporter Gene Insertion. Molecular and Cellular Biology, 2000, 20(11), 4106–4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7 -.Xu N, Li X, Zhong Y. Inflammatory Cytokines: Potential Biomarkers of Immunologic Dysfunction in Autism Spectrum Disorders. Mediators of Inflamm., 2015, 2015, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8 -.Vargas D, Nascimbene C, Krishnan C, Zimmerman A, Pardo C. Neuroglial activation and neuroinflammation in the brain of patients with autism. Annals of Neurol. 2004, 57(1), 67–81. [DOI] [PubMed] [Google Scholar]
- 9 -.Suzuki K, Sugihara G, Ouchi Y, Nakamura K, Futatsubashi M, Takebayashi K, Yoshihara Y, Omata K, Matsumoto K, Tsuchiya KJ, Iwata Y, Tsujii M, Sugiyama T, Mori N Microglial Activation in Young Adults With Autism Spectrum Disorder. JAMA Psych. 2013, 70(1), 49–58. [DOI] [PubMed] [Google Scholar]
- 10 -.Morgan J, Chana G, Pardo C, Achim C, Semendeferi K, Buckwalter J, Courchesne E, Everall IP. Microglial Activation and Increased Microglial Density Observed in the Dorsolateral Prefrontal Cortex in Autism. Biol. Psych, 2010, 68(4), 368–376. [DOI] [PubMed] [Google Scholar]
- 11 -.Lee A, Azmitia E, Whitaker-Azmitia P. Developmental microglial priming in postmortem autism spectrum disorder temporal cortex. Brain, Behavior, and Immunity, 2017, 62, 193–202. [DOI] [PubMed] [Google Scholar]
- 12 -.Velmeshev D, Schirmer L, Jung D, Haeussler M, Perez Y, Mayer S, Bhaduri A, Goyal N, Rowitch D, Kriegstein A. Single-cell genomics identifies cell type–specific molecular changes in autism. Science, 2019, 364(6441), 685–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13 -.Li Y, Zhang X, Li Y. Antineuroinflammatory therapy: potential treatment for autism spectrum disorder by inhibiting glial activation and restoring synaptic function. CNS Spectrums. 2019, 1–9. [DOI] [PubMed] [Google Scholar]
- 14 -.Wang Y, Zhao S, Liu X, Zheng Y, Li L, Meng S. Oxytocin improves animal behaviors and ameliorates oxidative stress and inflammation in autistic mice. Biomedicine & Pharmacotherapy, 2018, 107, 262–269. [DOI] [PubMed] [Google Scholar]
- 15 -.Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nature genetics, 1999, 23, (2), 185–188. [DOI] [PubMed] [Google Scholar]
- 16 -.Nagarajan R, Hogart A, Gwye Y, Martin MR, LaSalle JM. Reduced MeCP2 expression is frequent in autism frontal cortex and correlates with aberrant MECP2 promoter methylation. Epigenetics. 2006, 1, (4), 172–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17 -.Maezawa I, Jin L. Rett Syndrome Microglia Damage Dendrites and Synapses by the Elevated Release of Glutamate. Journal of Neuroscience, 2010, 30(15), 5346–5356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18 -.Derecki N, Cronk J, Lu Z, Xu E, Abbott S, Guyenet P, Kipnis J. Wild-type microglia arrest pathology in a mouse model of Rett syndrome. Nature, 2012, 484(7392), 105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19 -.Wang J, Wegener J, Huang T, Sripathy S, De Jesus-Cortes H, Xu P, Tran S, Knobbe W, Leko V, Britt J, Starwalt R, Mcdaniel L, Ward CS, Parra D, Newcomb B, Lao U, Nourigat C, Flowers DA, Cullen S, Jorstad NL, Yang Y, Glaskova L, Vigneau S, Kozlitina J, Yetman MJ, Jankowsky JL, Reichardt SD, Reichardt HM, Gärtner J, Bartolomei MS, Fang M, Loeb K, Keene CD, Bernstein I, Goodell M, Brat DJ, Huppke P, Neul JL, Bedalov A, Pieper AA. Wild-type microglia do not reverse pathology in mouse models of Rett syndrome. Nature, 2015, 521(7552), E1–E4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20 -.Zhao D, Mokhtari R, Pedrosa E, Birnbaum R, Zheng D, Lachman H. Transcriptome analysis of microglia in a mouse model of Rett syndrome: differential expression of genes associated with microglia/macrophage activation and cellular stress. Molecular Autism, 2017, 8(1), 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21 -.Cronk J, Derecki N, Ji E, Xu Y, Lampano A, Smirnov I, Baker W, Norris G, Marin I, Coddington N, Wolf Y, Turner S, Aderem A, Klibanov A, Harris T, Jung S, Litvak V, Kipnis J. Methyl-CpG Binding Protein 2 Regulates Microglia and Macrophage Gene Expression in Response to Inflammatory Stimuli. Immunity, 2015, 42(4), 679–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22 -.Schafer D, Heller C, Gunner G, Heller M, Gordon C, Hammond T, Wolf Y, Jung S, Stevens B. Microglia contribute to circuit defects in Mecp2 null mice independent of microglia-specific loss of Mecp2 expression. eLife, 2016, 5, e15224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23 -.Horiuchi M, Smith L, Maezawa I, Jin L. CX3CR1 ablation ameliorates motor and respiratory dysfunctions and improves survival of a Rett syndrome mouse model. Brain, Behavior, and Immunity, 2017, 60, 106–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24 -.Nance E, Kambhampati S, Smith E, Zhang Z, Zhang F, Singh S, Johnston MV, Kannan RM, Blue ME, Kannan S. Dendrimer-mediated delivery of N-acetyl cysteine to microglia in a mouse model of Rett syndrome. Journal of Neuroinflammation, 2017, 14(1), 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25 -.Deepmala, Slattery J, Kumar N, Delhey L, Berk M, Dean O, Spielholz C, Frye R. Clinical trials of N-acetylcysteine in psychiatry and neurology: A systematic review. Neuroscience & Biobehavioral Reviews. 2015, 55, 294–321. [DOI] [PubMed] [Google Scholar]
- 26 -.Hardan A, Fung L, Libove R, Obukhanych T, Nair S, Herzenberg L, Frazier T, Tirouvanziam R. A Randomized Controlled Pilot Trial of Oral N-Acetylcysteine in Children with Autism. Biological Psychiatry. 2012, 71, (11), 956–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27 -.Erny D, de Angelis AL, Jaitin D, Wieghofer P, Staszewski O, David E, Keren-Shaul H, Mahlakoiv T, Jakobshagen K, Buch T, Schwierzeck V. Host microbiota constantly control maturation and function of microglia in the CNS. Nature neuroscience, 2015, 18(7), 965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28 -.Lebovitz Y, Ringel-Scaia V, Allen I, Theus M. Emerging Developments in Microbiome and Microglia Research: Implications for Neurodevelopmental Disorders. Frontiers in Immunology, 2018, 9, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]