Figure 4. αsyn inclusion pathology assessed with N- and C-terminal specific αsyn antibodies is similar at motor impairment end stage of IM seeded M83+/− mice irrespective of the αsyn C-truncated hPFFs used for seeding.
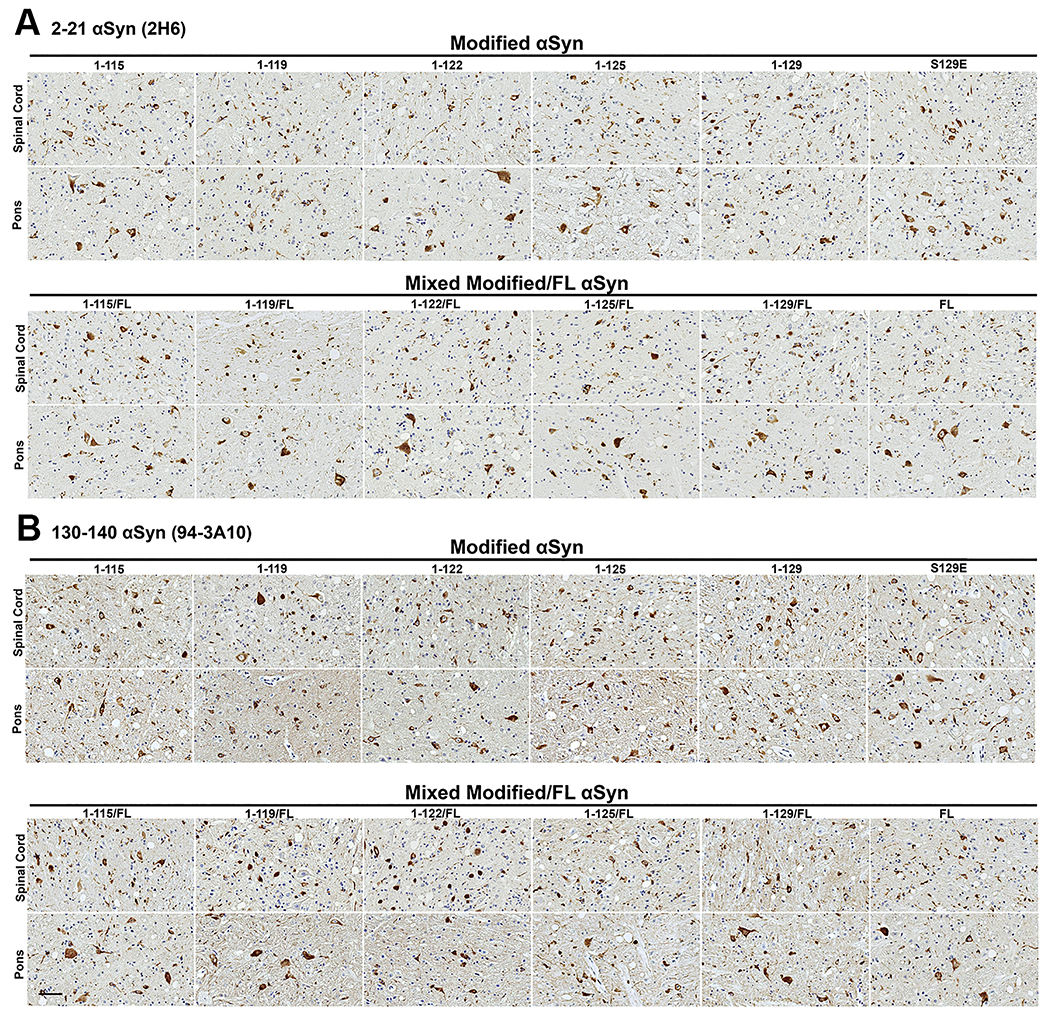
Representative immunohistochemical sections from the spinal cord and pons are shown from cohorts IM injected with hPFFs of varied compositions (1-115, 1-119, 1-122, 1-125, 1-129, S129E, and FL human αsyn or 1:1 mixed fibrils with each C-truncated form of αsyn and FL human αsyn). (A) Immunohistochemical staining with monoclonal antibody 2H6 (N-terminal epitope residues 2-21 αsyn) or (B) with monoclonal antibody 94-3A10 (C-terminal epitope residues 130-140 αsyn). Similar densities of neuritic and cellular αsyn inclusions are seen for all cohorts in the spinal cord and pons. Scale bar 50 μm.
