Abstract
Background
Improving surgeons’ technical performance may reduce their frequency of post-operative complications. We conducted a pilot trial to evaluate the feasibility of a surgeon-delivered audit and feedback intervention incorporating peer surgical coaching on technical performance among surgeons performing cleft palate repair, in advance of a future effectiveness trial.
Methods
A non-randomized, two-arm, unblinded pilot trial enrolled surgeons performing cleft palate repair. Participants completed a baseline audit of fistula incidence. Participants with a fistula incidence above the median were allocated to an intensive feedback intervention that included selecting a peer surgical coach, observing the coach perform palate repair, reviewing operative video of their own surgical technique with the coach, and proposing and implementing changes in their technique. All others were allocated to simple feedback (receiving audit results). Outcomes assessed were proportion of surgeons completing the baseline audit, disclosing their fistula incidence to peers, and completing the feedback intervention.
Results
Seven surgeons enrolled in the trial. All seven completed the baseline audit and disclosed their fistula incidence to other participants. The median baseline fistula incidence was 0.4% (range, 0%–10.5%). Two surgeons were unable to receive the feedback intervention. Of the five remaining surgeons, two were allocated to intensive feedback and three to simple feedback. All surgeons completed their assigned feedback intervention. Among surgeons receiving intensive feedback, fistula incidence was 5.9% at baseline and 0.0% following feedback (adjusted odds ratio, 0.98; 95% CI, 0.44–2.17).
Conclusions
Surgeon-delivered audit and feedback incorporating peer coaching on technical performance was feasible for surgeons.
Trial Registration
ClinicalTrials.gov identifier: NCT02583100
Introduction
The development of oronasal fistula after cleft palate repair is an important outcome to patients and families that can be objectively measured1–4 and depends in large part on technical skill.5,6 Fistula incidence is therefore a promising target for improvement efforts in cleft surgery. There is also substantial variation in incidence of fistula after palate repair, with rates of 0 to 35% reported in the literature.7 This variability in fistula incidence among surgeons, combined with the strong influence of technical skill on fistula incidence after palate repair, suggests that developing interventions to improve technical performance of cleft palate repair may reduce the incidence of postoperative fistula.
Formal interventions to improve surgeons’ technical performance are a new and promising direction for improving surgical outcomes.8 Outcomes vary among surgeons in numerous disciplines,9 and improving technical performance could lead to shorter operative times, lower complication rates, and reduced healthcare expenditures.10,11 Unfortunately, there is a paucity of evidence-based interventions that are effective at improving surgeons’ technical performance.
To address this unmet opportunity for improving surgical outcomes, investigators have begun exploring the effect of audit and feedback and the closely related concept of surgical coaching.12–16 Audit and feedback interventions collect standardized outcome measurements and report individual and peer-group results; they can improve performance in some settings, particularly when baseline performance is low, feedback is from trusted colleagues, and action plans are included.17 Surgical coaching is “a social interaction that aims to develop expertise by setting specific goals and providing feedback to achieve those goals.”18 Coaching can be a short, focused experience or involve frequent interactions over months or years.8,14 Combining audit and feedback with surgical coaching may be an effective approach to improving technical performance – providing rigorous measurement, meaningful goal setting, and actionable feedback delivered by a trusted and respected surgical peer.19 However, it is unclear whether a definitive trial evaluating the effectiveness of audit and feedback incorporating peer surgical coaching is feasible.18
We conducted a pilot trial to determine the feasibility of a future effectiveness trial for a surgeon-delivered audit and feedback intervention incorporating peer surgical coaching.20 Our primary objectives were to:
Determine whether surgeons would participate in the un-blinded disclosure of their fistula incidence among a group of their peers,
Determine whether surgeons would complete an intensive audit and feedback intervention that incorporated in-person visitation with a peer surgical coach, and
Identify barriers to completing a future effectiveness trial.
A secondary objective was to estimate the potential effect of the audit and feedback intervention on fistula incidence.
Methods
We conducted a non-randomized, two-arm, parallel group, unblinded pilot trial of a surgeon-delivered audit and feedback intervention with a 1:1 allocation ratio. The arms were (1) intensive feedback incorporating peer surgical coaching and (2) simple feedback allowing surgeons to make self-directed changes based on audit results.
The institutional review boards at all participating sites approved the study protocol. The study was registered at ClinicalTrials.gov ( NCT02583100). The CONSORT reporting guidelines for pilot and feasibility trials were followed.21
Participants
Attending plastic surgeons in the U.S. or Canada who performed ≥10 cleft palate repairs annually were recruited (9/2015–8/2016) from the Americleft Task Force Surgeon Subgroup (“Americleft”). The principal investigator (TJS) contacted eligible surgeons to discuss the study protocol, risks and benefits of participation, and eligibility criteria. Surgeons who completed the recruitment discussion and remained interested in participating provided informed written consent.
Interventions
Participating surgeons completed a baseline audit of their fistula incidence and documented their current operative technique by video recording three palate repairs.22,23 Audits prospectively evaluated all patients undergoing primary or secondary palate repair for the presence or absence of a fistula (any persistent hole between oral and mucosal surfaces located between the incisive foramen and the uvula base)3 at least 2 weeks post-surgery. Surgeons who had prospectively documented fistula occurrence on all patients before study enrollment were allowed to include up to 5 years of retrospective data. The baseline audit period was initially designed to include at least 9 months of prospectively evaluated operative cases, but delays in obtaining local ethics approval shortened this period at some centers. The cumulative period of prospective and retrospective case collection varied from 3 to 68 months among surgeons (median 24 months).
After the baseline audit period, surgeons unable to receive the intensive feedback intervention were excluded from participating in either intervention and reasons were documented. Participating surgeons with a fistula incidence above the median established by the baseline audit were assigned to receive intensive feedback. All others were assigned to receive simple feedback. Group assignment was not concealed.
Surgeons in both arms participated in a group conference call to discuss baseline audit results and review each surgeon’s fistula incidence in an unblinded fashion. Participants discussed data accuracy, and significance and possible causes of the differences in fistula incidence. Surgeons were then offered the opportunity to continue with their assigned feedback intervention or discontinue participation.
Following this conference call, surgeons in the simple feedback arm were given the opportunity to make self-directed changes in their surgical technique. Surgeons in the intensive feedback arm completed an evidence-based intervention designed to improve their performance using an in-person peer coaching session.17,19,24 First, they selected a peer surgical coach from the study participants whose fistula incidence was at or below the study’s median. Next, they completed a 2-day visit to their coach’s medical center during which they reviewed their own surgical technique with the coach using intraoperative videos recorded during the baseline period, directly observed the coach perform at least one palate repair live in the coach’s operating room, and observed the coach both in clinic and during ward rounds. With the coach’s assistance, participants then developed a personalized action plan containing up to three specific changes in their surgical technique or perioperative care. These planned changes were communicated in writing to the principal investigator (TJS). Participants then attempted to implement these changes during a 3-month implementation period. At the end of this implementation period, they met with the principal investigator who recorded whether planned changes were successfully implemented. The intensive feedback intervention was completed within 3 months of the baseline audit.
After the feedback interventions were complete, surgeons in both groups completed a post-feedback audit of their fistula incidence during the subsequent 9 months.
Outcomes
Willingness of surgeons to conduct a baseline audit, participate in unblinded disclosure of their individual fistula rate, and complete either simple or intensive feedback interventions was evaluated by proportion of surgeons completing these trial components. Barriers to completion of a future effectiveness trial were determined by documenting reasons for exclusion or withdraw of surgeons at any stage, and by reviewing investigators’ notes of events that delayed trial execution. The potential magnitude and effect of the intensive feedback intervention were determined by comparing fistula incidence at baseline and post-feedback within each study arm.
Sample Size
For this feasibility study, seven attending surgeons from six different institutions were recruited. This sample was believed to provide sufficient diversity to evaluate feasibility and to identify contextual and logistical barriers to completing a future trial of the intervention’s effectiveness.
For the secondary trial objective of estimating the potential direction and magnitude of effect of the intensive audit and feedback intervention on fistula incidence, the investigators calculated that seven surgeons performing 50 cleft palate repairs annually would provide 80% power to detect a 70% decrease from a baseline fistula rate of 10.4% to 3.1%, with a false-positive rate of 0.05. A baseline fistula rate of 10.4% was chosen because this was the historical fistula incidence at the trial coordinating center.25
Quantitative Analysis
For each palate operation, patient demographics (age, gender, race/ethnicity), cleft type, and palatal cleft width were obtained. Descriptive statistics for these patient characteristics were reported separately for the baseline audit and post-feedback periods.
Baseline fistula incidence for each surgeon was plotted using a funnel plot with three-sigma control limits.26 Fistula incidence for the baseline and post-feedback audits within study arm was subsequently reported with a 95% confidence interval (CI) calculated using adjusted Wald intervals.27 The potential magnitude and effect of the interventions were estimated using generalized estimating equations that adjusted for cleft width and allowed surgeon-specific random effects. Results were reported as odds ratios with 95% CI.
Qualitative Analysis
Investigators documented potential barriers to completing a future effectiveness trial as they were identified. This included actual challenges encountered during the feasibility study and experiences judged by the investigators to be potential barriers in a future trial. After the post-feedback audit, the list of potential barriers was grouped into categories, reviewed and agreed upon by the entire research team, and reported narratively.
Results
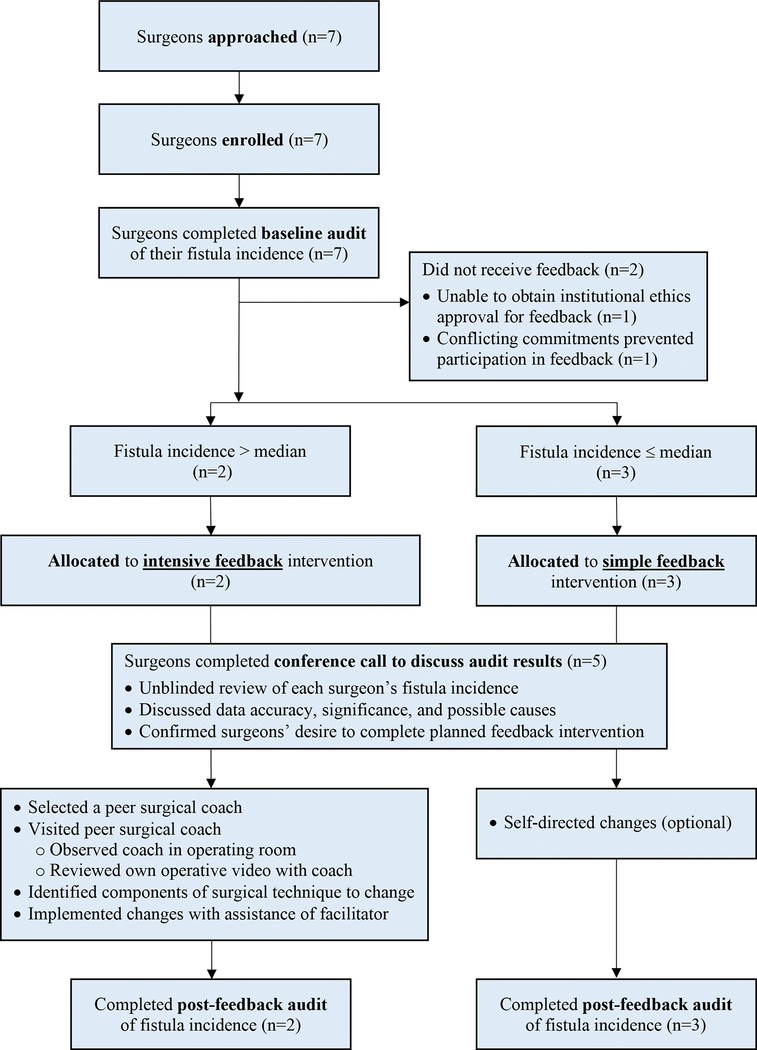
Figure 1 summarizes participant flow during the trial. Seven surgeons were approached for enrollment. All seven (100%) agreed to participate. All participants were members of a cleft team approved by the American Cleft Palate-Craniofacial Association (ACPA) and were actively involved in training plastic surgery residents. Six (86%) were employed at academic medical centers and one (14%) was in private practice. Surgeons were located in six different metropolitan areas across the U.S. and Canada. Additional practice characteristics are shown in Table 1. All surgeons employed a Furlow double opposing Z-plasty technique for Veau I clefts and a straight line mucosal incision with intra-velar veloplasty (IVVP) technique for Veau II, III, and IV clefts.
Figure 1:
Flowsheet summarizing the trial design and execution.
Table 1.
Surgeon participants.
| Participant Group | Surgeons in group, no. (%) | Cleft palate repairs performed annually, median (range) | Years in practice, median (range) |
|---|---|---|---|
| Intensive feedback | 2 (29) | 13 (10–15) | 4 (2–6) |
| Simple feedback | 3 (43) | 30 (28–47) | 16 (9–30) |
| Did not receive feedback | 2 (29) | 18 (17–19) | 14 (13–15) |
All seven surgeons completed the baseline audit of their fistula incidence. After the baseline audit, all seven surgeons confirmed their willingness to share audit results in an unblinded fashion with other participating surgeons.
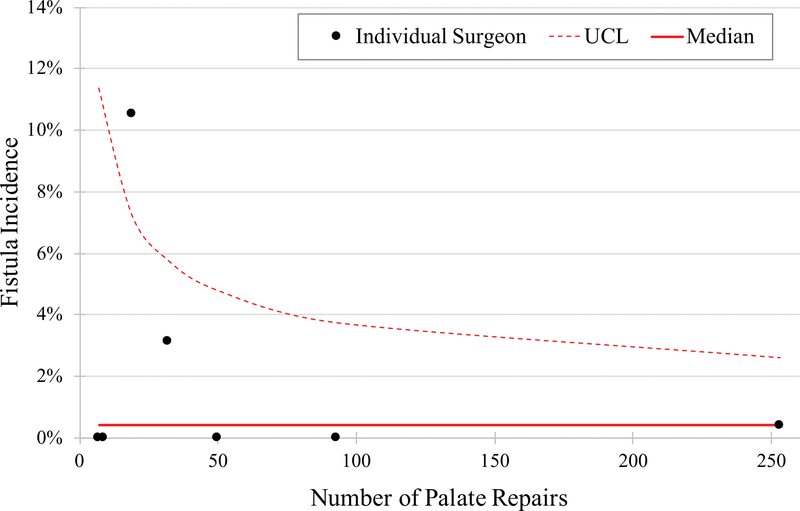
The median baseline fistula incidence was 0.4% (range, 0%–10.5%). The baseline fistula incidence for each participating surgeon is presented in Figure 2. Characteristics of patients undergoing cleft palate repairs during the trial are presented in Table 2.
Figure 2:
Funnel plot showing baseline fistula incidence by surgeon. UCL, upper confidence limit.
Table 2.
Characteristics related to patients undergoing cleft palate repairs.a
| Characteristics | Baseline period (N=369) | Post-feedback period (N=101) |
|---|---|---|
| Male | 205 (56) | 70 (69) |
| Race | ||
| White | 63 (17) | 44 (44) |
| Black | 9 (2) | 5 (5) |
| Asian | 71 (19) | 5 (5) |
| Other | 32 (9) | 13 (13) |
| Not reported | 194 (53) | 34 (34) |
| Hispanic | 309 (84) | 53 (52) |
| Adopted | 26 (7) | 2 (2) |
| Indication for surgery | ||
| Primary repair | 312 (85) | 96 (95) |
| Re-repair for VPI | 57 (15) | 5 (5) |
| Age, months, median (IQR) | ||
| Primary repair | 12 (11–14) | 13 (12–18) |
| Re-repair for VPI | 83 (70–115) | 61 (59–148) |
| Cleft type | ||
| Veau I–soft palate | 47 (13) | 12 (12) |
| Veau II–hard and soft palate | 103 (28) | 17 (17) |
| Veau III/IV–cleft lip and palate | 218 (59) | 69 (68) |
| Not reported | 1 (0.3) | 3 (3) |
| Cleft width,b mm, median (IQR) | 9 (7–12) | 10 (6–12) |
| Trial arm assigned to surgeon | ||
| Intensive feedback | 52 (14) | 23 (23) |
| Simple feedback | 310 (84) | 72 (71) |
| Did not receive feedback | 7c (2) | 6c (6) |
| No postoperative evaluation | 1 (0.3) | 2 (2) |
Data presented are number (%) of patients unless otherwise indicated.
Cleft width only reported for primary repairs.
Patient characteristics were not available for one of the two surgeons who did not complete the study.
Abbreviations: VPI, velopharyngeal insufficiency
After the baseline audit, two participants (29%) were excluded from further study participation because they were unable to receive the intensive feedback intervention; one had personal health issues arise and another was unable to secure ethics approval for the feedback portion of this trial.
Thus, five of seven surgeons (71%) entered the feedback portion of the trial. Using the median baseline fistula incidence as the cut-point, the two surgeons with fistula incidence above the median were assigned to the intensive feedback intervention, and the three surgeons at or below the median were assigned to the simple feedback intervention.
All five surgeons (100%) assigned to receive feedback interventions participated in the group conference call discussing results of the baseline audit and possible causes for differences between surgeons. The two surgeons assigned to the intensive feedback intervention both completed this intervention successfully; they both planned and successfully implemented changes in surgical technique and perioperative care (Table 3). The three surgeons assigned to the simple feedback intervention all completed this intervention successfully; none elected to change their palate repair technique or perioperative care. All five surgeons receiving feedback interventions completed the post-feedback audit of their fistula incidence.
Table 3.
Changes in surgical technique or perioperative care proposed by participants receiving intensive feedback.
| Change proposed | Outcome |
|---|---|
| Participant #1 | |
| 1. Use single hook instead of forceps when handling flaps | Implemented |
| 2. Use tapered instead of cutting needles when suturing mucosa | Implemented |
| 3. For elevation of nasal mucosa off hard palate, start posteriorly (with muscular dissection) and proceed anteriorly, rather than starting at palatal shelf and turbinate and then heading posteriorly | Implemented |
| Participant #2 | |
| 1. Use Mayfield headrest for positioning | Implemented |
| 2. Use WECK-CEL® pointed sponges soaked in 1:1000 epinephrine as needed for hemostasis | Implemented |
| 3. Elevate nasal mucosa off of the medial pterygoid plates | Implemented |
WECK-CEL® pointed sponges (Beaver Visitec International, Massachusetts)
The overall trial duration was 2 years. Enrollment began in September 2015. The feedback intervention was delivered September 2016–November 2016. The post-feedback audit was completed in August 2017.
Estimated Effect Size
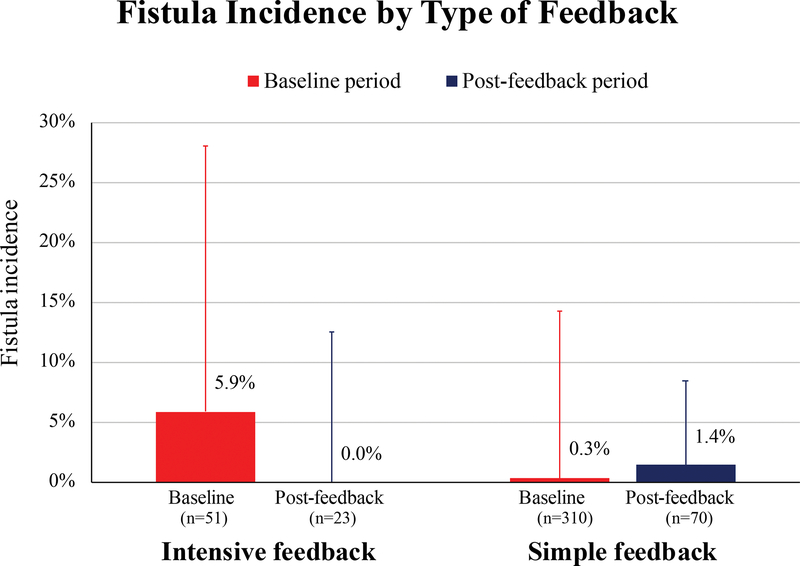
Among surgeons receiving intensive feedback, fistula incidence was 5.9% (3/51 repairs) during the baseline period and 0.0% (0/23 repairs) post-feedback (adjusted odds ratio, 0.98; 95% CI 0.44–2.17) (Figure 3). Among surgeons receiving simple feedback, fistula incidence was 0.3% (1/310 repairs) during the baseline period and 1.4% (1/70 repairs) post-feedback (adjusted odds ratio, 1.02; 95% CI 0.69–1.50).
Figure 3:
Fistula incidence by type of feedback, at baseline and after intervention. Bars represent group mean and lines represent standard deviation.
Potential Barriers
The study team identified three potential barriers to completing a definitive effectiveness trial. The first potential barrier was lower-than-expected fistula rates and cleft palate repair volume among participants. The overall fistula incidence during the study—1.1% (5/470)—was substantially lower than our a priori assumption. The number of palate repairs performed during the study period was also below historical levels for six of the seven participants. At study initiation, all participants provided an estimated target for annual palate repairs; only one surgeon met this target.
A second potential barrier was acquiring research ethics and regulatory approval. Ethics and regulatory staff at four of the six participating sites expressed concern that exposing surgeons to peer-delivered feedback could harm patients if surgeons adopted changes in technique that increased their complication rates. These concerns were satisfactorily addressed at all but one participating site by educating staff on quality assurance and improvement in healthcare and by implementing a safety monitoring process to continuously monitor fistula incidence. This monitoring process identified no adverse events among surgeons or patients related to study participation.
A third potential barrier was timely execution of the intensive feedback intervention. Based on existing evidence for optimal timing of feedback delivery, participants were to receive the surgical coaching within 3 months of completing the baseline audit. While this target was achieved, challenges were encountered in arranging participants’ travel dates to coincide with coaches’ scheduled surgeries.
Discussion
The results of our pilot trial indicate that surgeon-delivered audit and feedback incorporating peer surgical coaching can be successfully delivered to plastic surgeons performing cleft palate repair. These findings are consistent with reports showing that surgeons are willing to receive feedback on technical performance from a surgical coach.8 In a pilot study of four surgeons,15 performing a “post-game analysis” of complex laparoscopic procedures by reviewing intraoperative video with a senior surgical coach was considered very valuable. Similarly, video-based intraoperative coaching on technical performance was well received among surgeons learning laparoscopy.28 These findings among surgeons mirror findings of high acceptability for coaching among surgical trainees.29,30 Given the acceptability of coaching and evidence for improvement in technical skills afterward,30–40 further exploration of surgical coaching as an educational intervention is warranted.
Our findings extend prior studies of surgeon-directed audit and feedback and surgical coaching. First, they demonstrate that surgical coaching of practicing surgeons, previously applied to general surgeons15,40,41 and gynecologists,39 can be applied to plastic surgeons performing cleft palate repair. Second, they demonstrate that audit and feedback can be combined with surgical coaching, suggesting surgical coaching can target specific measurable objectives rather than overall improvement.42 Finally, they demonstrate that a single 2-day surgical coaching experience that incorporates video-based self-assessment of operative technique and observation of a coach performing the operation can help surgeons identify specific change to improve their operative technique. This 2-day coaching experience is distinct from the brief 1-hour “post-game analysis” format15 and the development of month- or year-long relationships evaluated in previous surgical coaching studies.39–41,43 These findings provide evidence that the participants, components, and duration of surgical coaching for practicing surgeons can be varied depending on the context and objectives while maintaining acceptability among surgeon participants. This variability is consistent with the broad conceptualization of coaching established by the International Coach Federation,44 although it does present challenges to identifying the most effective and efficient approaches to delivery surgical coaching.
Our results are directly applicable to surgeons performing palate repair, suggesting they are willing to audit their fistula incidence and receive feedback incorporating surgical coaching on their technical performance. Moreover, it appears that surgical coaching incorporating video-based assessment of operative technique and in-person visitation with a coach can help surgeons identify specific changes to improve technique. This type of instruction on technical performance is essential to improving surgical outcomes, yet it is rarely available to practicing surgeons. Given the positive reception in this study, and the positive impact of surgical coaching observed in previous studies,8 it seems appropriate to study the effect of surgical coaching in other plastic surgery domains where complication rates are directly affected by technical performance (e.g., microsurgery and breast reconstruction).45,46
During the study we identified three potential barriers to completion of an effectiveness future trial evaluating surgeon-delivered audit and feedback incorporating peer surgical coaching: delays in obtaining ethics approval, difficulty scheduling in-person coaching sessions when participants are primed to receive feedback, and low operative volume coupled with low fistula incidence. Delays in obtaining ethics approval could be preempted through a centralized approval process and education to ethics officials about the nature and inherent risks of continuing medical education. Difficulty scheduling in-person coaching sessions immediately following the audit results could be addressed by initiating the scheduling process earlier in the study. The third potential barrier, lower than expected rates of cleft palate repairs and fistula occurrence among study participants, is more difficult to address.
Low rates of cleft palate repair limit accuracy in estimating fistula incidence, while low rates of fistula occurrence limit the ability to distinguish between surgeons. One approach to address this potential barrier may be extending the baseline and post-feedback audit periods to collect more palate repairs. Another approach may be restricting enrollment to surgeons with a higher rate of cleft palate repairs, although this could exclude lower-volume surgeons with high fistula rates who may benefit from the intervention. A third alternative would be intentionally over-recruiting surgeons with higher fistula rates who may be more likely to benefit from the feedback intervention. Whichever approach is chosen, an additional pilot study is needed to evaluate the approach before initiating an effectiveness trial.
Limitations
Results of this pilot trial must be interpreted in the context of the study design.20 As the application of audit and feedback with peer surgical coaching on technical performance are new to most surgeons, we recruited participants from the Americleft Taskforce, a group established to compare surgical outcomes across cleft centers. Americleft surgeons may be more willing to perform audit and feedback and this could affect enrollment or execution of a trial in a different population of surgeons.18 To address this limitation, we recommend a larger pilot study to establish feasibility among a broader group of cleft surgeons before proceeding with an effectiveness trial.
This pilot trial was underpowered to detect a difference in fistula incidence between baseline and post-feedback time periods; this comparison, which would be performed during a future effectiveness trial of the audit and feedback intervention, will require a larger sample of surgeons and more palate repairs performed during the post-feedback period to be adequately powered. Similarly, this pilot study was not adequately powered to compare post-feedback fistula rates between the simple and intensive feedback arms, nor was it adequately powered to compare fistula rates by surgeon case volume, years in practice, or type of palate repair (i.e. straight line mucosal incision with IVVP versus Furlow double-opposing Z-plasty). These limitation are inherent to the pilot study design,47 which was focused on evaluating the feasibility of delivering the intervention. A future effectiveness trial of the audit and feedback intervention would need to be adequately powered to address these issues.
Finally, this study evaluated fistula rates, which is only one of the outcomes of interest after palate repair. Future studies of audit and feedback with peer surgical coaching on technical performance of cleft palate repair may wish to consider fistula incidence together with incidence of velopharyngeal insufficiency and facial growth.
Conclusion
This pilot study of surgeon-delivered audit and feedback incorporating peer surgical coaching found that surgeons performing cleft palate repair are willing to participate in this educational intervention and are capable of identifying new skills to improve surgical technique. However, a larger pilot study is needed before proceeding to an effectiveness trial, to determine if the present study’s findings are generalizable to all cleft surgeons and to confirm a sample of surgeons with sufficient surgical volume and fistula rate could be enrolled in an effectiveness trial. This pilot study also adds to the growing body of evidence that surgical coaching is an acceptable and effective method for improving technical performance.
Acknowledgments
Trial Funding: This trial was funded through a Cincinnati Children’s Hospital Medical Center Place Outcomes Research Award. TJS received additional support from the National Institute of Dental and Craniofacial Research (K23 DE025023). The funders had no role in the design of the study; the collection, analysis or interpretation of the data; the writing of the report; or the decision to submit the article for publication. The authors have no other financial relationships relevant to this article to disclose.
Financial Disclosure Statement: This trial was funded through a Cincinnati Children’s Hospital Medical Center Place Outcomes Research Award. TJS received additional support from the National Institute of Dental and Craniofacial Research (K23 DE025023). The funders had no role in the design of the study; the collection, analysis or interpretation of the data; the writing of the report; or the decision to submit the article for publication. The authors have no other financial relationships relevant to this article to disclose.
Bibliography
- 1.Allori AC, Kelley T, Meara JG, et al. A Standard Set of Outcome Measures for the Comprehensive Appraisal of Cleft Care. Cleft Palate Craniofac J. 2017;54(5):540–554. [DOI] [PubMed] [Google Scholar]
- 2.Paine KM, Paliga JT, Tahiri Y, et al. An Assessment of 30-Day Complications in Primary Cleft Palate Repair: A Review of the 2012 ACS NSQIP Pediatric. Cleft Palate Craniofac J. 2016;53(3):357–362. [DOI] [PubMed] [Google Scholar]
- 3.Sitzman TJ, Allori AC, Matic DB, et al. Reliability of Oronasal Fistula Classification. Cleft Palate Craniofac J. 2018:16186. [DOI] [PubMed] [Google Scholar]
- 4.Smith DM, Vecchione L, Jiang S, et al. The Pittsburgh Fistula Classification System: a standardized scheme for the description of palatal fistulas. Cleft Palate Craniofac J. 2007;44(6):590–594. [DOI] [PubMed] [Google Scholar]
- 5.Emory RE Jr., Clay RP, Bite U, Jackson IT. Fistula formation and repair after palatal closure: an institutional perspective. Plast Reconstr Surg. 1997;99(6):1535–1538. [PubMed] [Google Scholar]
- 6.Andersson EM, Sandvik L, Semb G, Abyholm F. Palatal fistulas after primary repair of clefts of the secondary palate. Scand J Plast Reconstr Surg Hand Surg. 2008;42(6):296–299. [DOI] [PubMed] [Google Scholar]
- 7.Hardwicke JT, Landini G, Richard BM. Fistula Incidence after Primary Cleft Palate Repair: A Systematic Review of the Literature. Plast Reconstr Surg. 2014;134(4):618e–627e. [DOI] [PubMed] [Google Scholar]
- 8.Min H, Morales DR, Orgill D, Smink DS, Yule S. Systematic review of coaching to enhance surgeons’ operative performance. Surgery. 2015;158(5):1168–1191. [DOI] [PubMed] [Google Scholar]
- 9.Birkmeyer JD, Finks JF, O’Reilly A, et al. Surgical skill and complication rates after bariatric surgery. N Engl J Med. 2013;369(15):1434–1442. [DOI] [PubMed] [Google Scholar]
- 10.Varban OA, Thumma JR, Finks JF, et al. Assessing variation in technique for sleeve gastrectomy based on outcomes of surgeons ranked by safety and efficacy: a video-based study. Surg Endosc. 2019;33(3):895–903. [DOI] [PubMed] [Google Scholar]
- 11.Varban OA, Thumma JR, Finks JF, Carlin AM, Ghaferi AA, Dimick JB. Evaluating the Effect of Surgical Skill on Outcomes for Laparoscopic Sleeve Gastrectomy: A Video-based Study. Ann Surg. 2019. [DOI] [PubMed] [Google Scholar]
- 12.Antoniou SA, Antoniou GA, Franzen J, et al. A comprehensive review of telementoring applications in laparoscopic general surgery. Surg Endosc. 2012;26(8):2111–2116. [DOI] [PubMed] [Google Scholar]
- 13.Hu YY, Peyre SE, Arriaga AF, Roth EM, Corso KA, Greenberg CC. War stories: a qualitative analysis of narrative teaching strategies in the operating room. Am J Surg. 2012;203(1):63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gawande A Personal Best. In. New Yorker; Vol 872011:44–53. [Google Scholar]
- 15.Hu YY, Peyre SE, Arriaga AF, et al. Postgame analysis: using video-based coaching for continuous professional development. J Am Coll Surg. 2012;214(1):115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greenberg CC, Ghousseini HN, Pavuluri Quamme SR, Beasley HL, Wiegmann DA. Surgical coaching for individual performance improvement. Ann Surg. 2015;261(1):32–34. [DOI] [PubMed] [Google Scholar]
- 17.Ivers N, Jamtvedt G, Flottorp S, et al. Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev 2012;6:CD000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mutabdzic D, Mylopoulos M, Murnaghan ML, et al. Coaching Surgeons: Is Culture Limiting Our Ability to Improve? Ann Surg. 2015;262(2):213–216. [DOI] [PubMed] [Google Scholar]
- 19.Beasley HL, Ghousseini HN, Wiegmann DA, Brys NA, Pavuluri Quamme SR, Greenberg CC. Strategies for Building Peer Surgical Coaching Relationships. JAMA Surg. 2017;152(4):e165540. [DOI] [PubMed] [Google Scholar]
- 20.Eldridge SM, Lancaster GA, Campbell MJ, et al. Defining Feasibility and Pilot Studies in Preparation for Randomised Controlled Trials: Development of a Conceptual Framework. PLoS One. 2016;11(3):e0150205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eldridge SM, Chan CL, Campbell MJ, et al. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. BMJ. 2016;355:i5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allori AC, Marcus JR, Daluvoy S, Bond J. Video-assisted palatopharyngeal surgery: a model for improved education and training. Cleft Palate Craniofac J. 2014;51(5):605–612. [DOI] [PubMed] [Google Scholar]
- 23.Demoss P, Murage KP, Tholpady S, Friel M, Havlik RJ, Flores RL. Low-cost, high-definition video documentation of corrective cleft surgeries using a fixed laparoscope. J Plast Reconstr Aesthet Surg. 2014;67(2):e58–59. [DOI] [PubMed] [Google Scholar]
- 24.Hu YY, Mazer LM, Yule SJ, et al. Complementing Operating Room Teaching With Video-Based Coaching. JAMA Surg. 2017;152(4):318–325. [DOI] [PubMed] [Google Scholar]
- 25.Pan BS, Rapp SJ, Vu A, Uribe-Rivera A, Billmire DA, Gordon CB. Evolution in minimal-incision palatoplasty: surgical technique and outcomes in 67 consecutive cases. Plast Reconstr Surg. 2014;134(1):102–111. [DOI] [PubMed] [Google Scholar]
- 26.Provost LP, Murray SK. The health care data guide : learning from data for improvement. 1st ed San Francisco, CA: Jossey-Bass; 2011. [Google Scholar]
- 27.Agresti A, Coull BA. Approximate Is Better than “Exact” for Interval Estimation of Binomial Proportions. The American Statistician. 1998;52(2):119–126. [Google Scholar]
- 28.Sereno S, Mutter D, Dallemagne B, Smith CD, Marescaux J. Telementoring for minimally invasive surgical training by wireless robot. Surg Innov 2007;14(3):184–191. [DOI] [PubMed] [Google Scholar]
- 29.Ahmed M, Arora S, Russ S, Darzi A, Vincent C, Sevdalis N. Operation debrief: a SHARP improvement in performance feedback in the operating room. Ann Surg. 2013;258(6):958–963. [DOI] [PubMed] [Google Scholar]
- 30.Zimmerman H, Latifi R, Dehdashti B, et al. Intensive laparoscopic training course for surgical residents: program description, initial results, and requirements. Surg Endosc. 2011;25(11):3636–3641. [DOI] [PubMed] [Google Scholar]
- 31.Cole SJ, Mackenzie H, Ha J, Hanna GB, Miskovic D. Randomized controlled trial on the effect of coaching in simulated laparoscopic training. Surg Endosc. 2014;28(3):979–986. [DOI] [PubMed] [Google Scholar]
- 32.Karam MD, Thomas GW, Koehler DM, et al. Surgical Coaching from Head-Mounted Video in the Training of Fluoroscopically Guided Articular Fracture Surgery. J Bone Joint Surg Am. 2015;97(12):1031–1039. [DOI] [PubMed] [Google Scholar]
- 33.Bonrath EM, Dedy NJ, Gordon LE, Grantcharov TP. Comprehensive Surgical Coaching Enhances Surgical Skill in the Operating Room: A Randomized Controlled Trial. Ann Surg. 2015;262(2):205–212. [DOI] [PubMed] [Google Scholar]
- 34.Singh P, Aggarwal R, Tahir M, Pucher PH, Darzi A. A randomized controlled study to evaluate the role of video-based coaching in training laparoscopic skills. Ann Surg. 2015;261(5):862–869. [DOI] [PubMed] [Google Scholar]
- 35.Greenberg CC, Dombrowski J, Dimick JB. Video-Based Surgical Coaching: An Emerging Approach to Performance Improvement. JAMA Surg. 2016;151(3):282–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Porte MC, Xeroulis G, Reznick RK, Dubrowski A. Verbal feedback from an expert is more effective than self-accessed feedback about motion efficiency in learning new surgical skills. Am J Surg. 2007;193(1):105–110. [DOI] [PubMed] [Google Scholar]
- 37.Mueller G, Hunt B, Wall V, et al. Intensive skills week for military medical students increases technical proficiency, confidence, and skills to minimize negative stress. J Spec Oper Med. 2012;12(4):45–53. [DOI] [PubMed] [Google Scholar]
- 38.Kirkpatrick JS. A comparison C1-C2 transarticular screw placement after self-education and mentored education of orthopaedic residents. J Spinal Disord Tech. 2012;25(6):E155–160. [DOI] [PubMed] [Google Scholar]
- 39.Briet JM, Mourits MJ, Kenkhuis MJ, van der Zee AG, de Bock GH, Arts HJ. Implementing an advanced laparoscopic procedure by monitoring with a visiting surgeon. J Minim Invasive Gynecol. 2010;17(6):771–778. [DOI] [PubMed] [Google Scholar]
- 40.Birch DW, Asiri AH, de Gara CJ. The impact of a formal mentoring program for minimally invasive surgery on surgeon practice and patient outcomes. Am J Surg. 2007;193(5):589–591; discussion 591–582. [DOI] [PubMed] [Google Scholar]
- 41.Schlachta CM, Sorsdahl AK, Lefebvre KL, McCune ML, Jayaraman S. A model for longitudinal mentoring and telementoring of laparoscopic colon surgery. Surg Endosc. 2009;23(7):1634–1638. [DOI] [PubMed] [Google Scholar]
- 42.Bannister SL, Wu TF, Keegan DA. The Clinical COACH: How to Enable Your Learners to Own Their Learning. Pediatrics. 2018;142(5). [DOI] [PubMed] [Google Scholar]
- 43.Shubeck SP, Kanters AE, Sandhu G, Greenberg CC, Dimick JB. Dynamics within peer-to-peer surgical coaching relationships: Early evidence from the Michigan Bariatric Surgical Collaborative. Surgery. 2018;164(2):185–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.International Coaching Federation. ICF - Frequently Asked Questions. Available at: https://coachfederation.org/faqs. Accessed July 10, 2019.
- 45.Heidemann LN, Gunnarsson GL, Salzberg CA, Sorensen JA, Thomsen JB. Complications following Nipple-Sparing Mastectomy and Immediate Acellular Dermal Matrix Implant-based Breast Reconstruction-A Systematic Review and Meta-analysis. Plast Reconstr Surg Glob Open. 2018;6(1):e1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berlin NL, Tandon VJ, Qi J, et al. Hospital Variations in Clinical Complications and Patient-reported Outcomes at 2 Years After Immediate Breast Reconstruction. Ann Surg. 2019;269(5):959–965. [DOI] [PubMed] [Google Scholar]
- 47.Kistin C, Silverstein M. Pilot Studies: A Critical but Potentially Misused Component of Interventional Research. JAMA. 2015;314(15):1561–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]