Fig. 1. Preparation of NSCs from 17 weeks’ human embryonic brain and OL specification.
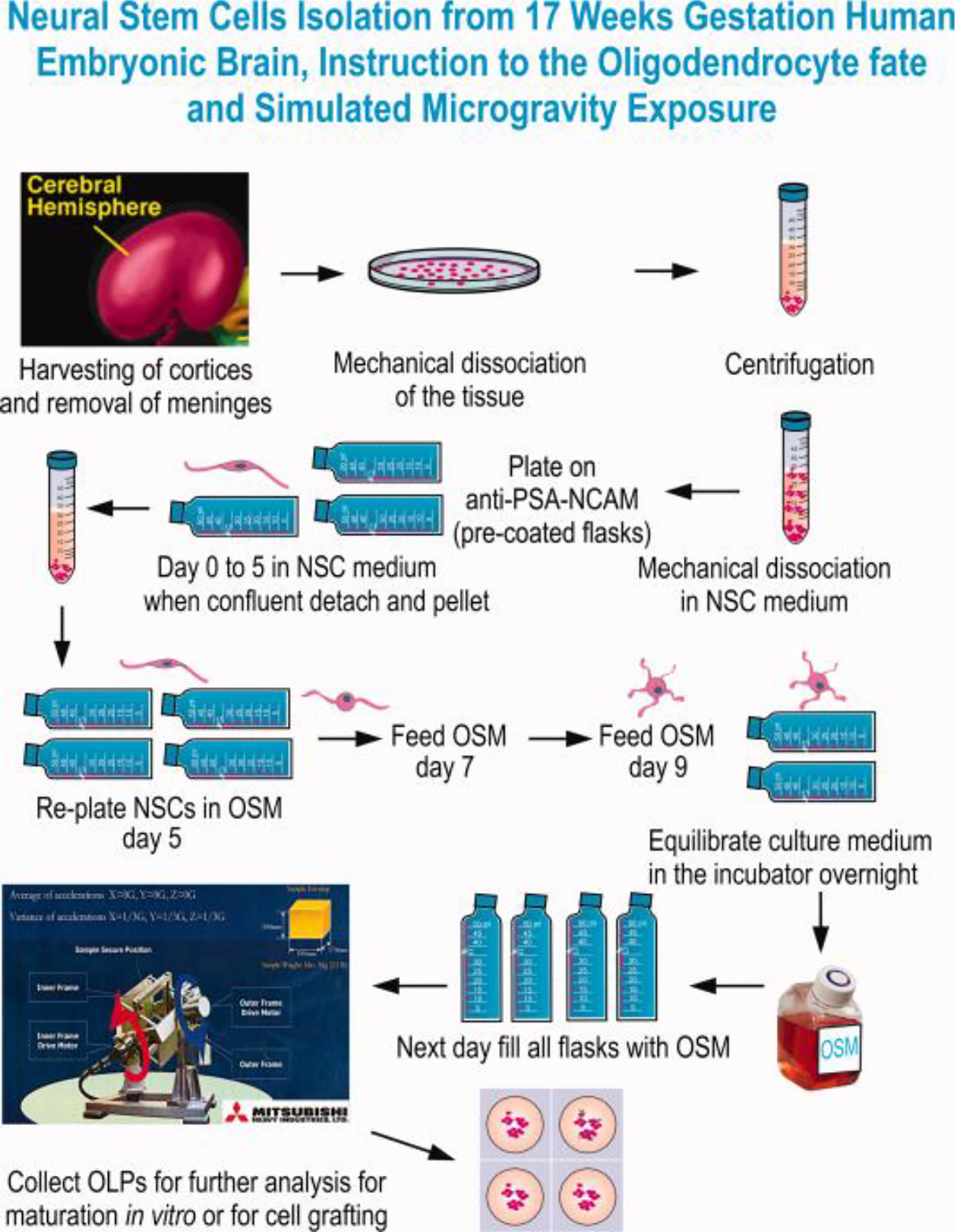
In sterile conditions and using the Biohazard safety cabinet, we started by dissecting the brain, harvested the cortices and remove the meninges. Following dissection and using STM the cell suspension was mechanically dissociated and pelleted. Cells were suspended in STMc medium (Espinosa et al., 2009; 2016). The cell suspension was sieved through a 70μm mesh and plated on anti-PSA-NCAM antibody–coated dishes. Cells were allowed to adhere for 5 min prior to removing them from the safety cabinet. NSCs were propagated directly on this matrix of PSA-NCAM antibody. To preserve self-renewal and stemness 50% of the medium was removed and replenished with the same volume of STMc every other day until confluent. When confluent, NSCs were harvested by shaking the flask, pelleted and seeded on PdL coated flasks with OSM. This medium instructs them towards the OL fate choice, while allowing them to proliferate. For both 1G and 0G cultures the incubator was set at 36.8°C and 4.5% CO2 as usual. For 1G and 0G cultures the full volume of culture medium was pre-equilibrated overnight in the CO2 incubator. To use the 3D-clinostat, the flasks or cell containers need to be full and without even a small bubble, in order to prevent demise of the cells. While this study was performed using 2D culture oligo spheres (3D cultures) would also work. After OSM has been equilibrated flasks were filled, the bubble removed and the cultures placed in the 3D-clinostat. The cells that would remain in 1G were placed in the bottom of the same incubator and using the same flasks, coating and containing the same volume of medium. Cultures were recovered after 72h for analysis.
