Figure 3: Nlrp3- and Asc-KO inhibits CaMKII-α and promotes phosphatase activity.
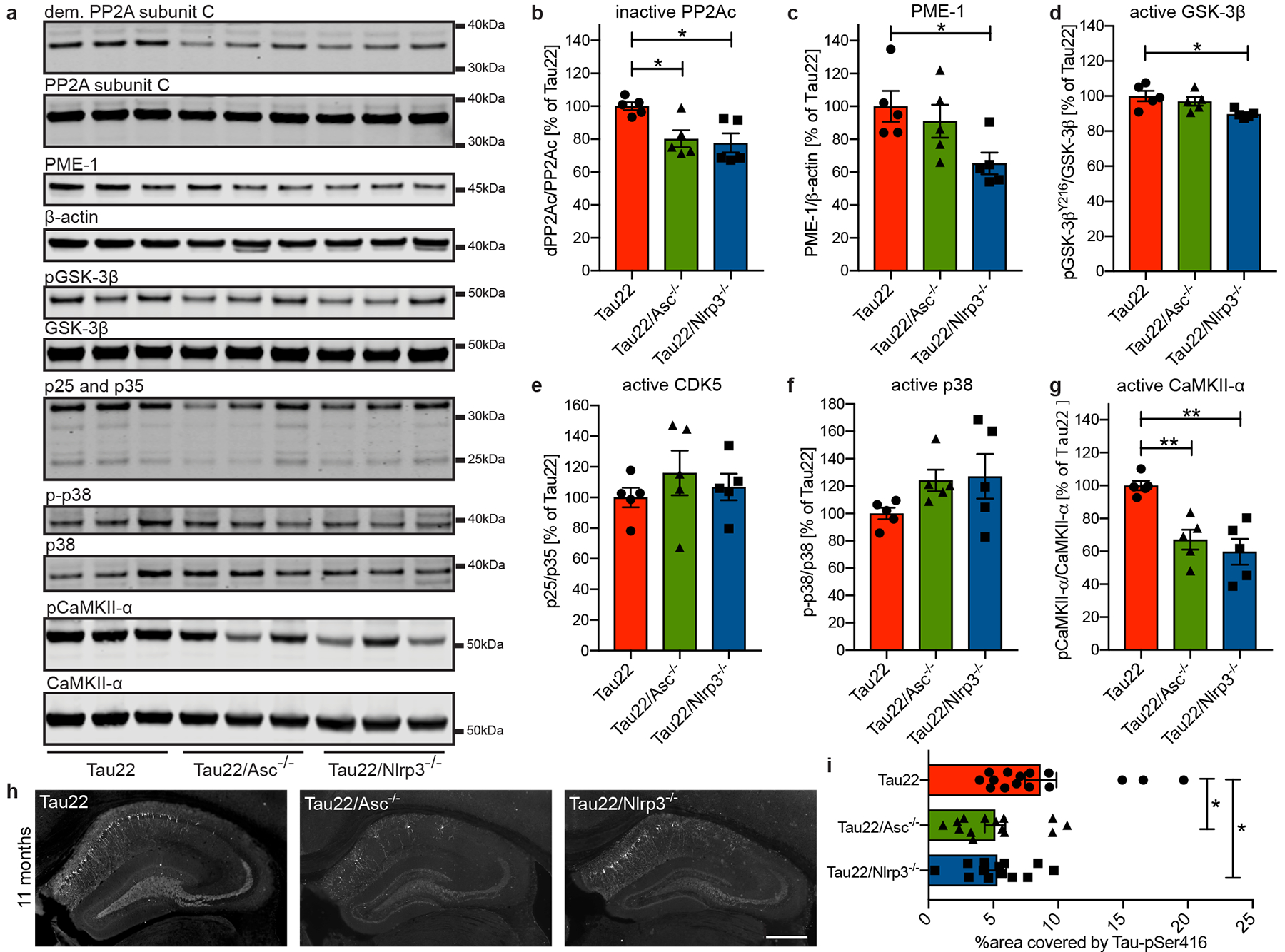
a, Immunoblot analysis of mouse hippocampi (11 months) stained for demethylated PP2A subunit C (dem. PP2A subunit C), total PP2A subunit C, PP2A methylesterase (PME-1), β-actin, GSK-3β phosphorylated at Tyr216 (pGSK-3β), total GSK-3beta, p25/p35, phosphorylated p38 (p-p38), total p38, Calmodulin dependent protein kinase II alpha phosphorylated at Thr286 (pCaMKII-α) and total CaMKII-α.
b - g, Quantification of the enzyme activities/abundance shown in a. n=5. PP2Ac: Tau22 vs. Tau22/Asc−/−: *P= 0.0286, Tau22 vs. Tau22/Nlrp3−/−: *P=0.0144, PME1: *P=0.0398, GSK-3β: *P=0.0205, CaMKII-α: Tau22 vs. Tau22/Asc−/−: **P=0.0055, CaMKII-α Tau22 vs. Tau22/Nlrp3−/−: **P=0.0012.
h, Immunohistochemical staining for Tau phosphorylated at Serin 416 (Tau-pSer416) in mouse hippocampi. Scale bar, 500μm.
i, Quantification of Tau-pSer416 in CA1 region shown in h. n=15 for Tau22, n=14 for Tau22/Asc−/−, n=13 for Tau22/Nlrp3−/−. Tau22 vs. Tau22/Asc−/−: *P=0.0314, Tau22 vs. Tau22/Nlrp3−/−: *P=0.0476.
For gel source data, see Supplementary Figure 1. Data are mean ± SEM and were analyzed by one-way ANOVA with Tukey’s test.
