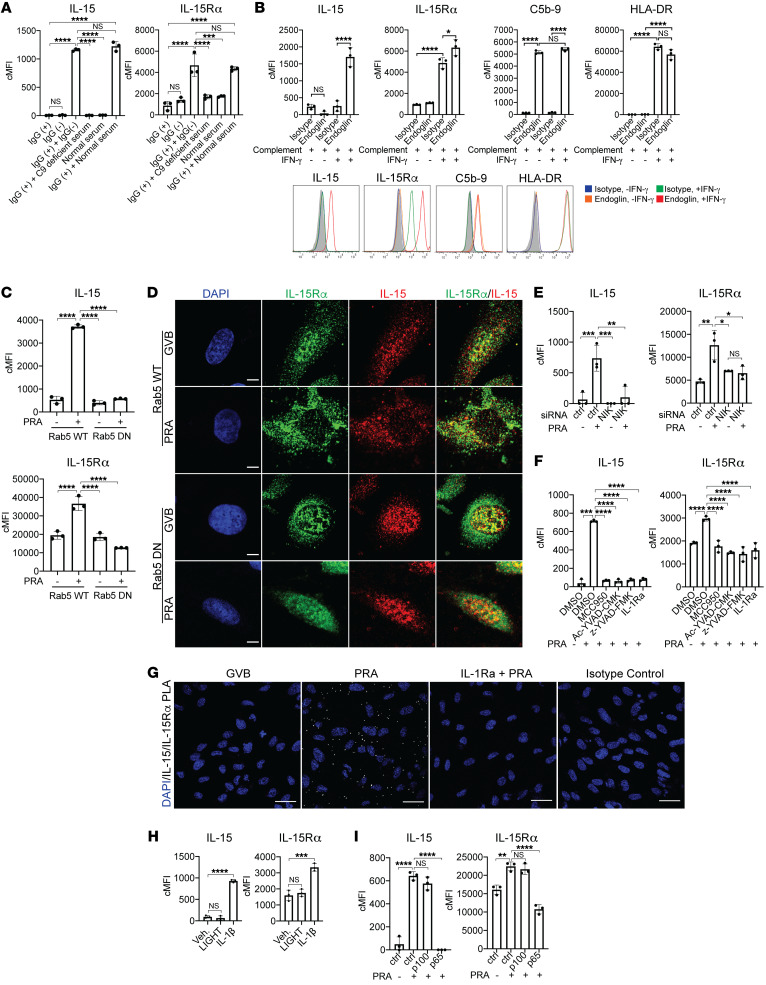
Figure 3. MAC stabilization of endosomal NIK, activation of NLRP3 inflammasome, and IL-1 signaling drives nuclear translocation and induction of IL-15/IL-15Rα complexes on human EC cell surfaces.
(A) PRA sera was separated into IgG+ and IgG− fractions and added to IFN-γ–pretreated ECs alone or in combination with C9-deficient or normal serum before flow cytometry analysis of surface IL-15 and IL-15Rα (n = 3). (B) Unprimed and IFN-γ–primed ECs were incubated with complement-fixing anti–human endoglin IgG2a antibody and human complement before flow cytometry analysis of IL-15, IL-15Rα, HLA-DR, and C5b-9 (n = 3). (C) IFN-γ–primed and stably transduced Rab5-WT or Rab5-DN (S43N) ECs were treated with PRA sera or control GVB and stained for surface IL-15 and IL-15Rα (n = 3). (D) IFN-γ–primed Rab5-WT and Rab5-DN ECs were treated with PRA and stained for intracellular IL-15 and IL-15Rα. Scale bars: 5 μm. (E) IFN-γ–pretreated ECs were transfected with NIK siRNA, treated with PRA, and analyzed for surface IL-15 and IL-15Rα (n = 3). (F) IFN-γ–primed ECs were pretreated with NLRP3 inhibitor MCC950, caspase-1 inhibitors Ac-YVAD-CMK or z-YVAD-FMK, or IL-1 receptor antagonist (IL-1Ra), treated with PRA and analyzed for surface IL-15 and IL-15Rα (n = 3). (G) IFN-γ–primed ECs were pretreated with either vehicle or IL-1Ra before PRA treatment. Proximity ligation assay (PLA) was performed between surface IL-15 and IL-15Rα and analyzed by confocal microscopy. Scale bars: 30 μm. (H) IFN-γ–primed ECs were treated with LIGHT, IL-1β, or mock treatment before analysis of surface IL-15 and IL-15Rα (n = 3). (I) IFN-γ–primed ECs were transfected with control, p100, or p65 siRNA before PRA treatment and flow cytometry analysis for surface IL-15 and IL-15Rα (n = 3). Data represent mean ± SEM. **P < 0.01; ****P < 0.0001; 1-way ANOVA and Tukey’s multiple comparisons test. Representative of 3 independent experiments using 3 HUVEC donors.