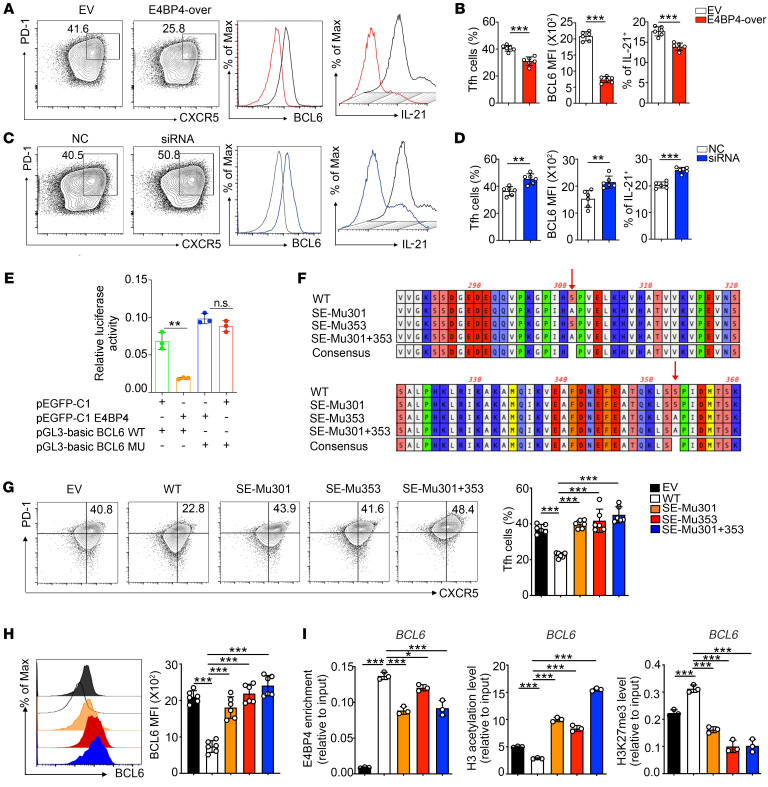
Figure 7. Phosphorylation-defective mutant of E4BP4 promotes human Tfh cell differentiation.
(A–D) CD4+ T cells isolated from healthy donors were transfected with E4BP4 overexpression (E4BP4-over) plasmid or empty vector (EV), as well as with E4BP4 siRNA and negative control (NC). The cells were then cultured under Tfh polarization conditions for 5 days. (A) Flow cytometric analysis of CD4+CXCR5+PD-1+ Tfh cells in EV and E4BP4-over group. Representative histograms of BCL6 and IL-21 expressions in CD4+CXCR5+PD-1+ Tfh cells are shown on the right. (B) Statistical analysis of A. (C) Analysis of CD4+CXCR5+PD-1+ Tfh cells in NC and siRNA groups. Representative histograms of BCL6 and IL-21 expressions in CD4+CXCR5+PD-1+ Tfh cells are shown. (D) Statistical analysis of C (n = 6). (E) The luciferase reporter assay in HEK293T cells by electroporation with the reporter plasmids pEGFP-C1 empty vector plus pGL3-basic BCL6 WT or pEGFP-C1 E4BP4 plus pGL3-basic BCL6 WT or pEGFP-C1 E4BP4 plasmid plus pGL3-basic BCL6 mutant or pEGFP-C1 empty vector plasmid plus pGL3-basic BCL6 mutant (n = 3). (F) Phosphomimetic mutation of E4BP4 putative serine sites at ser301 or ser353 mutants or double mutants (S to A). (G) Mutant and WT versions of E4BP4 were expressed in human CD4+ T cells, separately. Cells were then cultured under Tfh-polarizing conditions for 5 days and the percentage of CD4+CXCR5+PD-1+ Tfh cells was analyzed by flow cytometry. (H) BCL6 MFI expression in CD4+CXCR5+PD-1+ Tfh cells indicated in G was analyzed by flow cytometry (n = 6). (I) Mutant and WT versions of E4BP4 were expressed in Jurkat cells. ChIP-PCR was used to detect levels of E4BP4 enrichment, H3 acetylation, and H3K27 trimethylation in the BCL6 gene promoter (n = 3). Data are representative of 3 independent experiments. For B, D, and E, Student’s t tests were used. For G–I, 1-way ANOVA analysis with Dunnett’s post hoc test was used. *P < 0.05; **P < 0.01; ***P < 0.001.