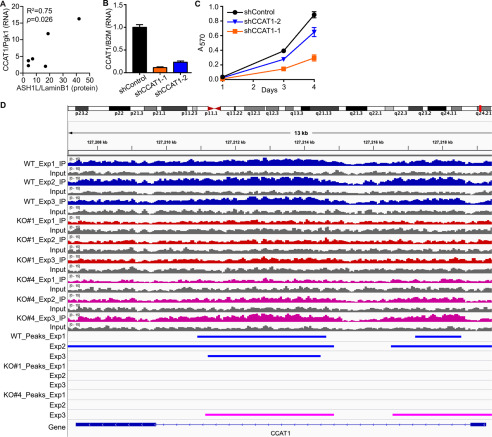
Figure 5.
CCAT1 is an ASH1L target gene and a mediator of ASH1L function. A, ASH1L protein expression normalized to lamin B1 (data from Fig. 1) was compared with CCAT1 lncRNA expression in the same tumors, measured by RT-qPCR and normalized to Pgk1. The expression of CCAT1 correlates with that of ASH1L protein (R2 = 0.75; p = 0.026). B, two independent shRNAs were used to knock down CCAT1 expression in BHT-101 cells, compared with shControl cells. CCAT1 lncRNA levels were determined by RT-qPCR, normalized to B2M, and expressed relative to the shControl value, which was set to 1. The bars indicate means ± S.D. (n = 3). Both shCCAT1-1 and -2 differ significantly from shControl (p < 0.0001; Dunnett's test). C, BHT-101 cells with CCAT1 knockdown grow more slowly than shControl cells, assessed by MTT assay. Each data point represents the means ± S.D. for sextuplicate wells. Both shCCAT1 cell lines differ significantly from shControl at days 3 and 4 (p < 0.0001, Dunnett's test). D, CCAT1 is a direct target gene of ASH1L. ChIP-Seq was performed on BHT-101 WT cells and the ASH1L KO clones 1 and 4 using an antibody for H3K36me2, the histone mark catalyzed by ASH1L. In 3 separate experiments, a portion of the CCAT1 gene (marked by blue bars) is dimethylated at H3K36 in the WT cells (WT), but the H3K36me2 peaks are lost in all 3 experiments in the KO#1 cell line and in 2 of 3 experiments in the KO#4 cell line (KO#4 experiment 3 peaks are indicated by pink bars). Peaks were called using MACS2 (FDR < 0.05).