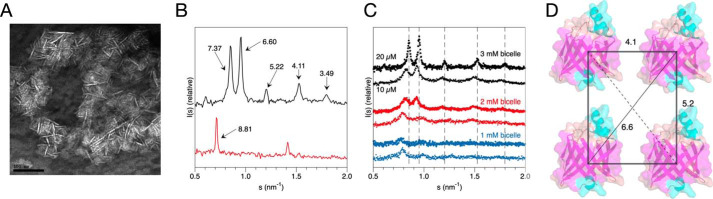
Figure 2.
Insights into P2 structure between membranes from bicelle complexes. A, negative staining EM micrograph of P2-stacked bicelles. Being flat lipid discs, the bicelles are here mainly seen as thin lines, corresponding to a side view. Scale bar, 100 nm. B, Bragg X-ray diffraction peaks from P2-stacked bicelles (black) and vesicles (red). The corresponding repeat distances are marked. C, titration of protein and lipid concentration in the bicelle samples indicates shorter distances and higher order when both protein and lipid concentrations increase. D, a model of P2 arrangement on the plane of the membrane, based on the peak positions in B. Note that the 7.37-nm peak corresponds to the bicelle membrane stack repeat distance, and the 3.49-nm distance could be a sign of an additional tighter arrangement of P2 on the membrane, because a 3.5-nm distance was seen in cryo-EM. For the vesicle sample in B, the 8.81-nm peak corresponds to the stack repeat between consecutive membrane layers, and no information is obtained on the lateral protein arrangement.