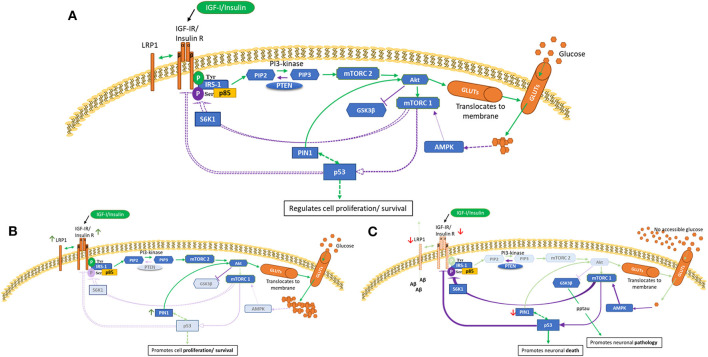
Figure 1.
PI3K/Akt pathway in health (A), cancer (B) and AD brain (C) cells. This is a schematic of the PI3K/Akt cellular pathway which regulates cell proliferation, metabolism and death. These figures attempt to highlight possible differences in cancer and AD compared with health. These indicated differences, as described in human and animal tissues and in cell culture, are meant to represent general concepts not specific cases. (A) shows normal regulation (B) indicates a cancer phenotype (C) illustrates AD as an insulin-resistant state i.e., T3DM. Green lines represent activation and purple lines represent feedback from the activation pathway. Activation of the IGF-1/insulin receptors leads to tyrosine phosphorylation of IRS-1 and activation of mTORC2 and Akt, resulting in glucose uptake. Homeostasis is maintained partly by mTORC1 sensing of metabolic conditions, which, as appropriate, leads to phosphorylation of p53 and S6K1 serine phosphorylation of IRS-1. p53 is a negative regulator of IGF/insulin receptors, IGF-II and glucose transporters. [A] Normal cellular homeostasis as described above [B] In cancer, negative feedback pathways are switched off leading to upregulation of proliferation, metabolism and cell survival. A modified genetic landscape (e.g., p53, PTEN) enables tumor cells to benefit from a glucose-rich, IGF/insulin-rich environment (insulin-resistance such as in T2DM).In cancer, Akt can phosphorylate and inactivate GSK-3β, which results in increased protein synthesis that supports cell growth. [C] In AD brain with insulin-resistance, or if, due to decreased blood flow there is no glucose accessible, the PI3K/Akt pathway is effectively switched off or downregulated. This leads to upregulation of GSK-β that culminates in tau phosphorylation and aggregation and increased amyloid beta production. Lack of intraneuronal glucose would trigger AMPK to activate mTORC1, p53, S6K1 serine phosphorylation of IRS-1. This could be a self-perpetuating cycle.