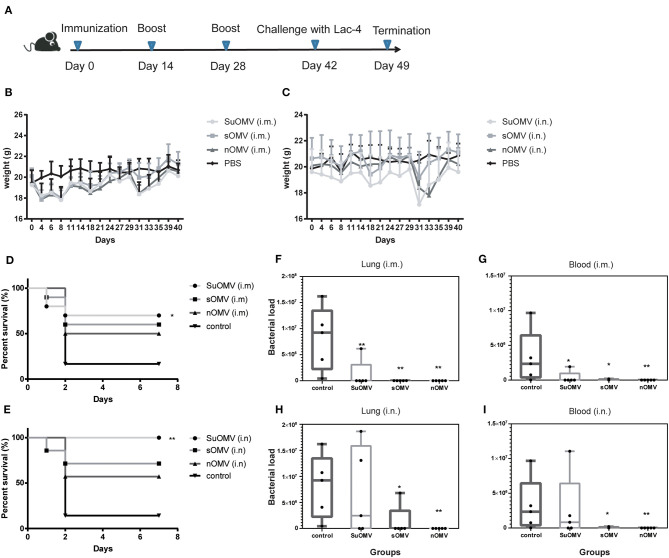
Figure 5.
Protective effect of AbOMVs against lethal and sub-lethal A. baumannii challenge. (A) Timeline representation of immunization schedule and experimental procedures. BALB/c mice were intramuscularly or intranasally immunized with either of the AbOMVs. Controls were treated with PBS. Two weeks after the final vaccination, mice were challenged intratracheally with A. baumannii Lac-4. (B,C) Weight changes in mice over the duration of vaccination were recorded for preliminary safety evaluation. (D,E) 7-days survival rates of immunized mice after lethal challenge were calculated (n = 10). Bacterial loads in lungs (F,H) and blood (G,I) of immunized mice 24 h post sub-lethal i nfection were determined by CFU counts of serial 10-fold dilutions on TSA. Dots show values in each mouse, boxes show the range for each group (min, median, and max), n = 5. *P < 0.05, **P < 0.01, compared with PBS control.