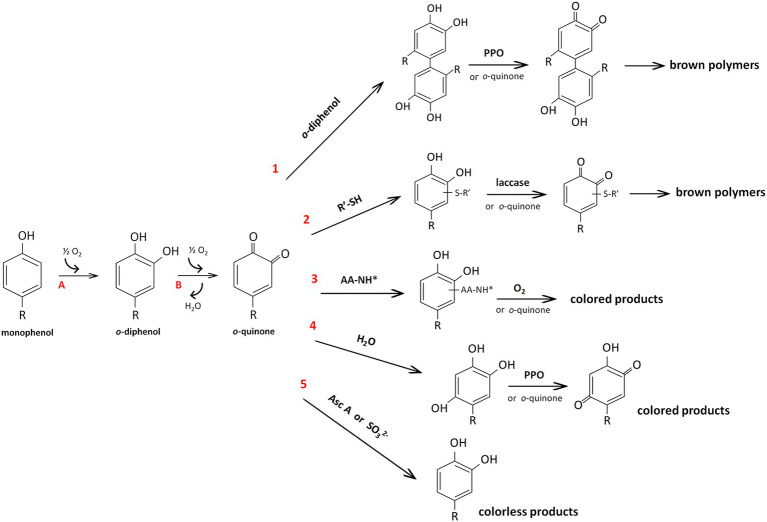
Figure 2.
Reactions catalyzed by polyphenol oxidase (PPO) (A, and B) and reactions of o-quinone (1–6) according to Nicolas et al. (1994). Due to monophenolase (or cresolase) and diphenolase (or catecholase) activity, PPOs hydroxylate monophenols to o-diphenols (A) and subsequently oxidize o-diphenols to o-quinones (B), respectively. The resulting o-quinones can react with another molecule of phenol with the formation of dimers of the original phenol (reaction 1). These dimers with an o-diphenolic structure can be oxidized either enzymatically or by another o-quinone to a brown polymer. By nucleophilic addition, o-quinones can interact with thiol groups (reaction 2) or amino groups of amino acids or peptides (reaction 3), resulting in compounds with an o-diphenolic structure that can be further oxidized (by laccase or oxygen) or react with an excess of o-quinones to form colored products. Water can be added to o-quinones, leading to triphenols that can be oxidized by PPO or by o-quinones with the formation of p-quinones (reaction 4). Finally, the reactions with ascorbic acid or sulfites lead to the regeneration of the original phenol (reaction 5). All reactions are nonenzymatic except for those with laccase and PPO. AA-NH*, amino acids or peptides; Asc A, ascorbic acid; R’-SH, small thiol compounds (e.g., cysteine or glutathione).