In their recently published study in Respiratory Physiology and Neurobiology, the authors (Arias-Reyes et al., 2020) attempted to explain available epidemiological data of Tibet and high-altitude regions of Bolivia and Ecuador that indicated that high-altitude inhabitants (+2500 m above sea-level) compared to lowland data sets, are less susceptible to develop severe adverse effects in acute SARS-CoV-2 virus infection. They suggest that physiological acclimatization/adaptation that counterbalance the hypoxic environment in high-altitude communities may protect from severe impact of acute SARS-CoV-2 virus infection.
The authors suggest that two potential underlying mechanisms may be responsible for these findings including: (i) a compromised half-live of the virus caused by the high altitude environment itself, and (ii) a hypoxia mediated down regulation of angiotensin-converting enzyme 2 (ACE2), as the main binding target of SARS-CoV-2 virus in the pulmonary epithelium
The authors list environmental factors that may influence the virulence of SARS-CoV-2 at high-altitude including drastic changes in temperature between night and day, air dryness and high levels of ultraviolet (UV) light radiation. They highlight the fact that UV light radiation A (UVA) and B (UVB) are known to produce alterations in the molecular bonds of the DNA and RNA, and thus UV radiation at high-altitude may act as a natural sanitizer.
While it is clear that UV radiation and multiple factors could dramatically reduce the “survival” capacity of the virus at high-altitude, the authors have excluded the role of ozone (O3) which is a well known natural disinfecting agent which can be found in very high concentrations in many high altitude environments. These high concentrations are the result of the downward push of stratospheric ozone rich air and the vertical uplift of air masses carrying pollutants from the heavily urbanized areas of South Asia and Indo-Gangetic Plain. In our work we have shown that surface ozone measurements increased with altitude with concentrations that can exceed 100 ppb (8 -h exposure). Highest values were during the spring season and the result of diverse contributions: hemispheric background values, the descent of ozone rich stratospheric air and the transport of tropospheric pollutants occurring at different spatial scales. These findings are common in most mountain major ranges in the world (Semple et al., 2016; Semple and Moore, 2009; Moore and Semple, 2009).
The reactivity of O3 is through oxidation, peroxidation or generation of free radicals and giving rise to cascade of reactions like peroxidation of lipids leading to changes in membrane permeability. In viruses, the O3 damages the viral capsid and upsets the reproductive cycle by disrupting the virus-to-cell contact with peroxidation (Elvis and Ekta, 2011; Gérard and Sunnen, 2003).
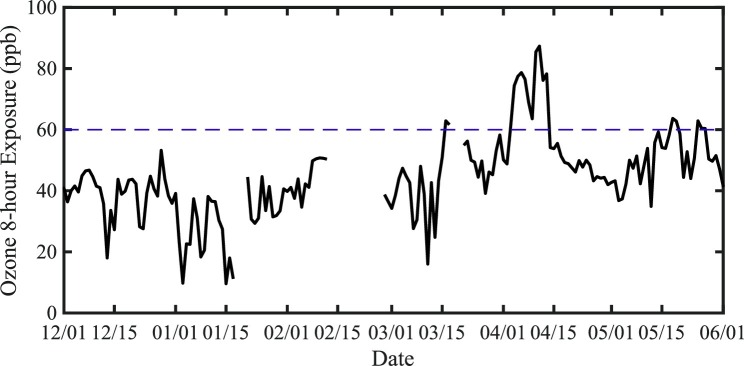
The health effects of ambient ozone exposures have been examined in many low elevation geographic regions. Potential adverse effects reported include decrements in lung function, airway inflammation, symptoms of asthma, increased rates of hospitalization and mortality for those with preexisting respiratory disease (Jerrett et al., 2009; Semple et al., 2016). In Katmandu during the months of the COVID-19 Pandemic despite the regional and international lockdown the ozone (03) levels were quite high even at lower altitudes (Fig. 1 ). The World Health Organization (WHO) recommends stricter guidelines than the EPA standards, 60 ppb over 8 h not to be exceeded more than 20 days in one year (Ozone (O3) Air Quality Standards - United-States-Environmental-Protection-Agency, 2015).
Fig. 1.
Time series of the surface 8 -h ozone exposure (ppb) as observed at the U.S. Embassy in Kathmandu (elevation 1400 m asl) December 1 2019- June 1 2020. The WHO guideline for 8 h exposure (60 ppb) is indicated by the dashed line.
Exertion at high altitude already has multiple factors that potentially contribute to adverse pulmonary effects including cold, dryness, hypoxia and decreased barometric pressure (Semple et al., 2016). Air quality in mountainous regions has been reported to worsen with increasing elevation due to other factors. In the Himalayas, wood and yak-dung stoves are common heat sources resulting to “in-door” pollution and so the air quality in villages is often poor, both indoor and outdoor, in the early morning and evening (Moore and Semple, 2009). Increasing elevation also leads to more intense solar radiation which, as the authors point out, can effect both the virus directly as well as producing accelerated photochemistry and greater ozone production from precursor elements (Semple et al., 2016; Moore and Semple, 2009).
Here we draw attention to the high altitudes communities where they are exposed to significant levels of ozone air pollution (well above WHO and EPA standards).
One can suggest there may be numerous environmental factors influencing the half-life of SARS-CoV-2 virus but having an oxidizing agent such as O3 present at high concentrations in many high altitude communities could be another way to explain the results found here.
Funding
No financial support was received for the conduct of the research and/or preparation of this article.
Declaration of Competing Interest
Authors declare that there are no conflicts of interest' in this article.
References
- Arias-Reyes C., Zubieta-DeUrioste N., Poma-Machicao L., Aliaga-Raduan F., Carvajal-Rodriguez F., Dutschmann M., Schneider-Gasser E.M., Zubieta-Calleja G., Soliz J. Does the pathogenesis of SARS-CoV-2 virus decrease at high-altitude? Respir. Physiol. Neurobiol. 2020;277 doi: 10.1016/j.resp.2020.103443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elvis A.M., Ekta J.S. Ozone therapy: a clinical review. J. Nat. Sci. Biol. Med. 2011;2(1):66–70. doi: 10.4103/0976-9668.82319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gérard V., Sunnen M.D. 2003. SARS and Ozone Therapy: Theoretical Considerations. [cited in 2003]http://www.triroc.com/sunnen/topics/sars.html Available from: [Google Scholar]
- Jerrett M., Burnett R.T., Pope C.A., III Long-term ozone exposure and mortality. N. Engl. J. Med. 2009;360:1085–1095. doi: 10.1056/NEJMoa0803894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore G.W.K., Semple J.L. High concentration of surface ozone observed along the Khumbu Valley Nepal April 2007. Geophys. Res. Lett. 2009;36 doi: 10.1029/2009gl038158. [DOI] [Google Scholar]
- Ozone (O3) Air Quality Standards - United-States-Environmental-Protection-Agency., 2015) https://www.epa.gov/naaqs/ozone-o3-air-quality-standards-documents-review-completed-2015.
- Semple J.L., Moore G.W.K. Ozone exposure and mortality. N. Engl. J. Med. 2009;360:2786–2787. doi: 10.1056/NEJMc090738. [DOI] [PubMed] [Google Scholar]
- Semple J.L., Moore G.W., Koutrakis P., Wolfson J.M., Cristofanelli P., Bonasoni P. High concentrations of ozone air pollution on Mount Everest: health implications for Sherpa communities and mountaineers. High Alt. Med. Biol. 2016;(October) doi: 10.1089/ham.2016.0042. [DOI] [PubMed] [Google Scholar]