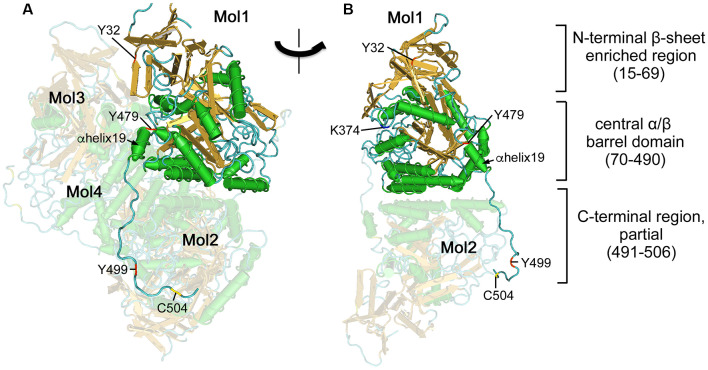
Figure 1.
Ternary structure of Collapsin response mediator proteins 2 (CRMP2). (A) Crystal structure of human CRMP2 (1–525) homo-tetramer (5X1A; Niwa et al., 2017). A secondary structure-based view was rendered using Cn3D (version 4.3). One monomer (Mol1) is shown in secondary structure-based color (α-helix, green; β-sheet, gold; random coil, cyan). The structure is visible between 14 and 506 residues. Phosphorylation (Tyr32, Tyr479, and Tyr499), SUMOylation (Lys374), and oxidation (Cys504) sites are indicated by red, blue, and yellow, respectively. Other monomers (Mol2–4) are shown in faded colors. The C-terminal domain of Mol1 interacts with Mol2. (B) Rotate view. The interface of Mol1 to Mol3 is shown by omitting Mol3 and Mol4. Note that Lys374 and Tyr479 are present on the interface.