Abstract
Aims:
To report trends in antibiotic resistance in cases of bacterial keratitis from a large eye hospital in South India.
Methods:
In this retrospective cross-sectional study, the microbiology laboratory records of patients with infectious keratitis diagnosed at an eye hospital in South India from 2002–2013 were reviewed to determine the proportion with antibiotic non-susceptibility.
Results:
3685 bacterial isolates had susceptibility testing performed over the 12-year period. The two most common organisms with resistance results were Streptococcus pneumoniae (N=1204) and Pseudomonas aeruginosa (N=894). Antibiotic non-susceptibility was generally uncommon for these two organisms, and no significant trends were detected over the course of the study. In contrast, Staphylococcus aureus (N=211) isolates demonstrated a significant increase in fluoroquinolone non-susceptibility over the 12-year study period. This coincided with a significant increase in methicillin-resistant S. aureus (MRSA) during the study period, though the increase in fluoroquinolone resistance was likewise seen in methicillin-sensitive S. aureus (MSSA). For example, ofloxacin resistance in MSSA increased from 11.1% in 2002 to 66.7% in 2013 (P=0.002). No trends were apparent for the aminoglycocides, cefazolin, or vancomycin, for which in vitro non-susceptibility generally appeared to be low.
Conclusions:
Resistance to antibiotics was generally stable for infectious keratitis isolates from a large eye hospital in South India, except for S. aureus, which experienced a significant increase in fluoroquinolone resistance from 2002 to 2013. Fluoroquinolone antibiotics currently have poor in vitro activity against both methicillin-resistant and methicillin-sensitive S. aureus in South India and are therefore not the ideal therapy for Staphylococcal corneal ulcers.
Keywords: corneal ulcer, bacteria, drug resistance, bacterial, fluoroquinolones
INTRODUCTION
Corneal ulceration is a leading cause of blindness worldwide. The incidence of corneal ulceration is estimated to be between 113 and 799 per 100,000 person years in south Asia—at least ten-fold higher than in the United States.[1–3] Treatment for corneal infections is based on appropriate antimicrobial therapy, which requires knowledge of the local antimicrobial susceptibility patterns of various antibiotics. There are reasons to think that this susceptibility profile may have changed over recent years. For example, oral antibiotic consumption has increased in India recently, and especially the use of cephalosporins has increased.[4 5] This increased antibiotic consumption may have selected for resistant strains of bacteria, and therefore changed the susceptibility profile. Despite the burden of corneal ulcers in India, recent surveillance data from the Indian subcontinent is lacking. In this study, we investigate for trends in antimicrobial resistance in corneal ulcers seen at a large tertiary care eye hospital in South India.
METHODS
We reviewed the records of the Microbiology Laboratory at the Aravind Eye Hospital Madurai to identify all bacterial cultures performed for corneal ulceration from 2002 to 2013. Aravind Eye Hospital Madurai is the largest eye hospital in the state of Tamil Nadu in South India, and serves both a primary care and referral population. Corneal ulcers were routinely scraped and plated on blood, chocolate, and potato dextrose agar. Blood and chocolate plates were incubated at 37°C and examined for growth daily for 7 days; potato dextrose plates were incubated at 27°C and examined daily for 3 weeks. Anaerobic cultures were not routinely performed. Bacterial isolates from corneal scrapings were tested for susceptibility to a panel of antibiotics with a disc diffusion assay (Himedia, India) according to the manufacturer’s recommendations, using resistance breakpoints according to guidelines from the Clinical and Laboratory Standards Institute (CLSI) or from the European Committee on Antimicrobial Susceptibility Testing (EUCAST) if CLSI guidelines were not available.[6] CLSI and EUCAST recommend an MIC assay for vancomycin resistance in Staphylococci, but this was not performed due to limited resources. Instead, a disc diffusion assay was performed for vancomycin resistance in Staphylococci, using the breakpoints from viridans group Streptococci. CLSI currently recommends inferring beta-lactam susceptibility from the results of oxacillin susceptibility testing. However, because cefazolin is a major therapy used for corneal ulcers, a disc diffusion assay for cefazolin was performed for Gram-positive isolates, using the CLSI breakpoint from Enterobacteriacea for all tested organisms. No CLSI guidelines exist for gatifloxacin resistance in non-urinary Pseudomonas aeruginosa, so breakpoints for Enterobacteriacea were used for determining gatifloxacin resistance in P. aeruginosa. The CLSI also does not provide resistance breakpoints for the disc diffusion assay for Nocardia or diphtheroids, so for these organisms we used the same breakpoints that we used for Staphylococci.
We depicted the proportion of bacterial cultures non-susceptible (i.e., resistance or intermediate resistance) to each of the tested antibiotics as the moving average, incorporating the prior and subsequent year’s numbers. We tested for differences in non-susceptibility between different antibiotics using an equality of proportions test. We tested for trends over time with Poisson regression, accounting for autocorrelation with time series bootstrap with a fixed width of two (tsboot package in R; 1000 repetitions; significance level of 0.05). Statistical analysis was done with the statistical software R 3.1.2. We obtained ethical approval for this study from the Aravind Institutional Review Board.
RESULTS
Of 17,948 patients with corneal ulcers who had cultures performed over the 12-year period, 3,615 (20.1%) had bacterial growth, 6218 (34.6%) had fungal growth, 261 (1.5%) had parasite growth, 113 (0.6%) had both bacterial and fungal growth, and the remainder (43.1%) had no growth on culture. Of 4,132 total bacterial isolates, 3,685 (89.2%) had susceptibility testing performed. The most commonly isolated bacteria with susceptibility results were Streptococcus pneumoniae (N=1204, 32.7%), Pseudomonas aeruginosa (N=894, 24.3%), Nocardia species (N=246, 6.7%), diphtheroids (N=235, 6.4%), viridans group Streptococcus (N=212, 5.8%), Staphylococcus aureus (N=197, 5.3%), coagulase negative Staphylococci (N=151, 4.1%), and Enterobacteriaceae (N=110, 3.0%); Table 1. The results of susceptibility testing are shown for each of the 12 years of the study in Supplementary Table 1 for Gram-positive organisms and Supplementary Table 2 for Gram-negative organisms; results for individual organisms are summarized below.
Table 1. Number of organisms with susceptibility test results each year over the 12-year study period.
Testing for methicillin-resistant S. aureus started in 2005.
| Organism | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gram-positive | ||||||||||||
| S. pneumoniae | 55 | 52 | 86 | 59 | 89 | 148 | 121 | 107 | 124 | 97 | 137 | 129 |
| Viridans group Streptococci | 5 | 14 | 18 | 21 | 18 | 9 | 18 | 23 | 24 | 15 | 22 | 25 |
| Methicillin-resistant S. aureus | --- | --- | --- | 0 | 0 | 0 | 1 | 4 | 2 | 5 | 5 | 3 |
| Methicillin-sensitive S. aureus | 9 | 11 | 10 | 5 | 16 | 8 | 21 | 23 | 18 | 19 | 22 | 15 |
| Coagulase negative Staphylococci | 7 | 10 | 14 | 20 | 15 | 4 | 7 | 8 | 10 | 13 | 24 | 19 |
| Nocardia species | 9 | 12 | 12 | 12 | 13 | 29 | 32 | 24 | 21 | 22 | 35 | 25 |
| Diphtheroids | 8 | 17 | 16 | 18 | 34 | 22 | 23 | 20 | 17 | 23 | 16 | 21 |
| Gram-negative | ||||||||||||
| P. aeruginosa | 52 | 50 | 58 | 74 | 82 | 84 | 107 | 66 | 97 | 80 | 73 | 71 |
| Enterobacteriaceae | 10 | 7 | 9 | 3 | 9 | 6 | 6 | 12 | 13 | 12 | 13 | 10 |
S. pneumoniae
Pneumococcal isolates from corneal ulcers generally remained susceptible to most antibiotics over the study period (Figure 1). Ofloxacin non-susceptibility was observed in 54 of 1201 (4.5%, 95%CI 3.4–5.8%) isolates over the entire study period; non-susceptibility was similar for other fluoroquinolones. Non-susceptibility to chloramphenicol was unusual (37 of 1204 isolates; 3.1%, 95%CI 2.2–4.2%) and vancomycin non-susceptibility was never observed (0%, 95%CI 0–0.3%). Thresholds for pneumococcal non-susceptibility to ciprofloxacin are not provided by the CLSI, but ciprofloxacin-resistant pneumococci were common when using the EUCAST criteria (290 of 1196 isolates; 24.2%, 95%CI 21.8–26.8%). Screening pneumococcal isolates for oxacillin resistance started in 2009; non-susceptibility was observed in 82 of 580 (14.1%, 95%CI 11.4 to 17.2%) isolates. Using the Enterobacteriacea breakpoint for cefazolin resistance, non-susceptibility was found in 5 of 1204 (0.4%, 95%CI 0.1 to 1.0%) isolates. Resistance to each of the tested antibiotics was stable over the 12-year duration of the study.
Figure 1. Trends in antibiotic resistance among Gram-positive organisms.
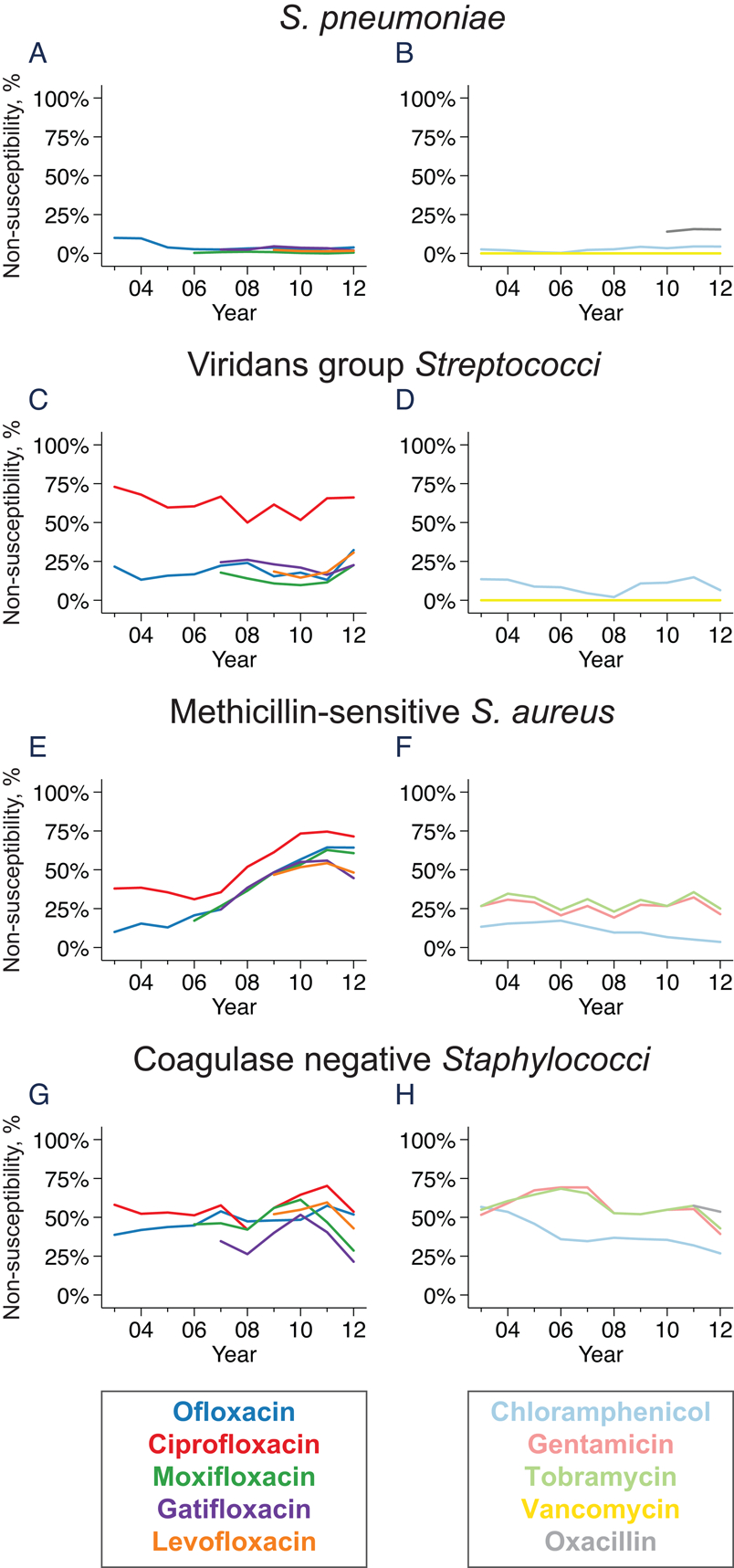
The graphs represent the moving average of the annual proportion of non-susceptible isolates for S. pneumoniae (parts A and B), viridans group Streptococci (parts C and D), methicillin-susceptible S. aureus (parts E and F), and coagulase negative Staphylococci (parts G and H).
P. aeruginosa
Ofloxacin non-susceptibility was seen in 116 of 887 (13.1%, 95%CI 10.9 to 15.5%) Pseudomonas corneal ulcers throughout the study period (Figure 2), with similar rates of non-susceptibility among the other tested fluoroquinolones. Non-susceptibility was similar for the aminoglycocides; for example, 121 of 894 (13.5%, 95%CI 11.4 to 16.0%) isolates were resistant to gentamicin over the study period. Non-susceptibility to ceftazidime was less common (36 of 651 isolates resistant; 5.5%, 95%CI 3.9 to 7.6%); P<0.001 compared with ofloxacin or gentamicin non-susceptibility. Resistance patterns were relatively stable over the duration of the study for all tested antibiotics.
Figure 2. Trends in antibiotic resistance among Gram-negative organisms.
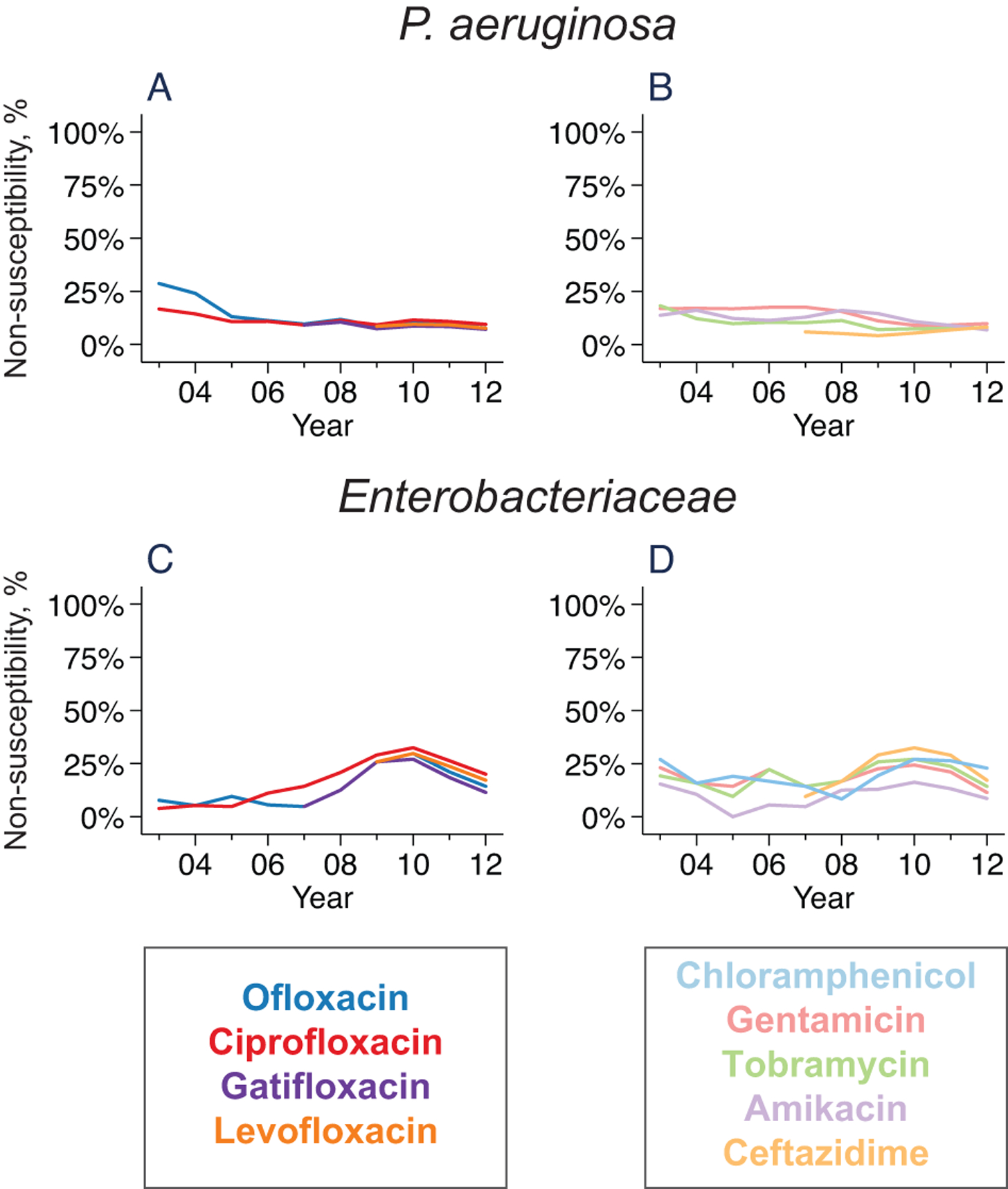
Lines represent the moving average of the proportion of non-susceptible isolates for P. aeruginosa (parts A and B) and Enterobacteriaceae (parts C and D).
Nocardia species
Resistance in Nocardia species was estimated from the disc diffusion assay using CLSI guidelines for Staphylococci. As shown in Figure 3, moderate levels of non-susceptibility were observed for all tested antibiotics except for amikacin (0 of 246 isolates classified as resistant; 95%CI 0–1.5%). Using the Enterobacteriacea breakpoint, 209 of 241 (86.7%, 95%CI 81.8 to 90.7%) Nocardia isolates were non-susceptible to cefazolin. Using the viridans group Streptococci breakpoint, 134 of 201 (66.7%, 95%CI 59.7 to 73.1%) were non-susceptible to vancomycin. No trends in antibiotic non-susceptibility were apparent for the study duration.
Figure 3. Trends in antibiotic resistance among Nocardia (parts A and B) and diphtheroids (parts C and D).
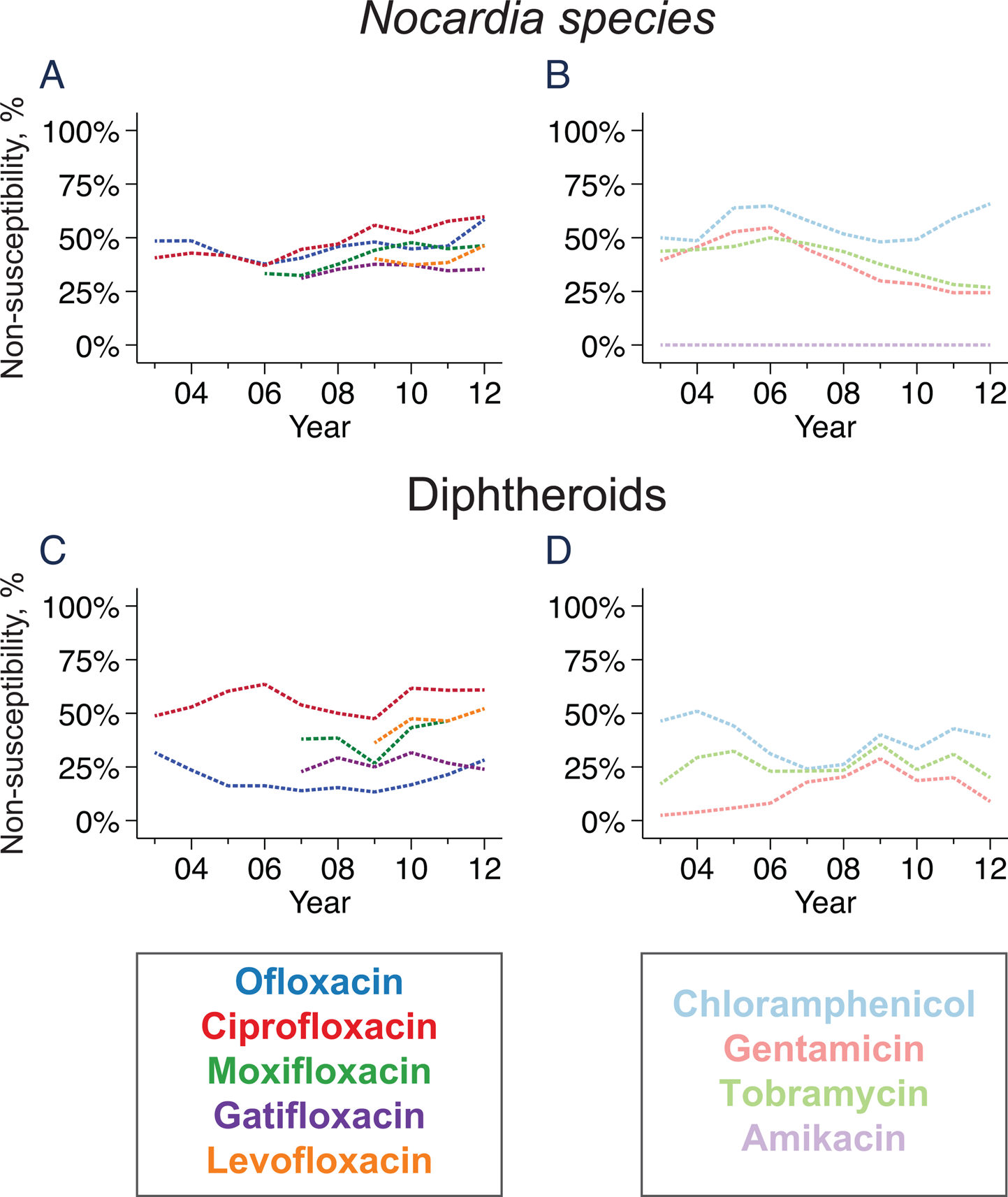
Lines represent the moving average of the proportion of isolates resistant to each antibiotic; lines are dashed to indicate that established breakpoints for the disc diffusion assay do not exist for either organism.
Diphtheroids
Resistance thresholds for diphtheroids are not available from CLSI, so we used the CLSI criteria for Staphylococci. Using these criteria, non-susceptibility to ofloxacin was seen in 46 of 221 (20.8%, 95%CI 15.7 to 26.8%) isolates, which was significantly less than each of the other fluoroquinolones except for gatifloxacin (P<0.001 when compared to ciprofloxacin, moxifloxacin, or levofloxacin; P=0.54 when compared to gatifloxacin). Using the Enterobacteriacea breakpoint for cefazolin resistance and viridans group Streptococci breakpoint for vancomycin resistance, non-susceptibility was found in 51 of 221 (23.1%, 17.7 to 29.2%) and 5 of 221 (2.3%, 95%CI 0.7 to 5.2%) diphtheroid isolates, respectively. Resistance did not markedly change over the study duration for any of the tested antibiotics (Figure 3).
Viridans group Streptococci
Fluoroquinolone non-susceptibility was more common for viridans group Streptococci than it was for S. pneumonia, with ofloxacin non-susceptibility observed in 46 of 212 (21.7%, 95%CI 16.3 to 27.9%) isolates (Figure 1; P<0.001 compared with proportion of S. pneumoniae non-susceptible to ofloxacin). Chloramphenicol non-susceptibility was uncommon and vancomycin non-susceptibility was never observed. Using the Enterobacteriacea breakpoint for cefazolin resistance, non-susceptibility was found in 12 of 212 (5.7%, 95%CI 3.0 to 9.7%) isolates.
Methicillin resistant S. aureus (MRSA)
We identified MRSA using the oxacillin disk diffusion assay starting in 2005. MRSA was not present from 2005 until 2007, but accounted for 1 of 22 (4.5%) S. aureus isolates in 2008, 4 of 27 (14.8%) isolates in 2009, 2 of 20 (10.0%) isolates in 2010, 5 of 24 (20.8%) isolates in 2011, 5 of 27 (18.5%) isolates in 2012, and 3 of 15 (20.0%) in 2013 (P=0.002 testing for a trend over time). Most of the MRSA isolated during the study period was also resistant to the fluoroquinolone and aminoglycoside antibiotics (Figure 4). Only a single isolate was resistant to chloramphenicol. No isolates were non-susceptible to vancomycin when classified using the breakpoints for viridans group Streptococci.
Figure 4. Spectrum of resistance in the 20 methicillin resistant Staphylococcus aureus isolates identified at Aravind Eye Hospital, 2007–2013.
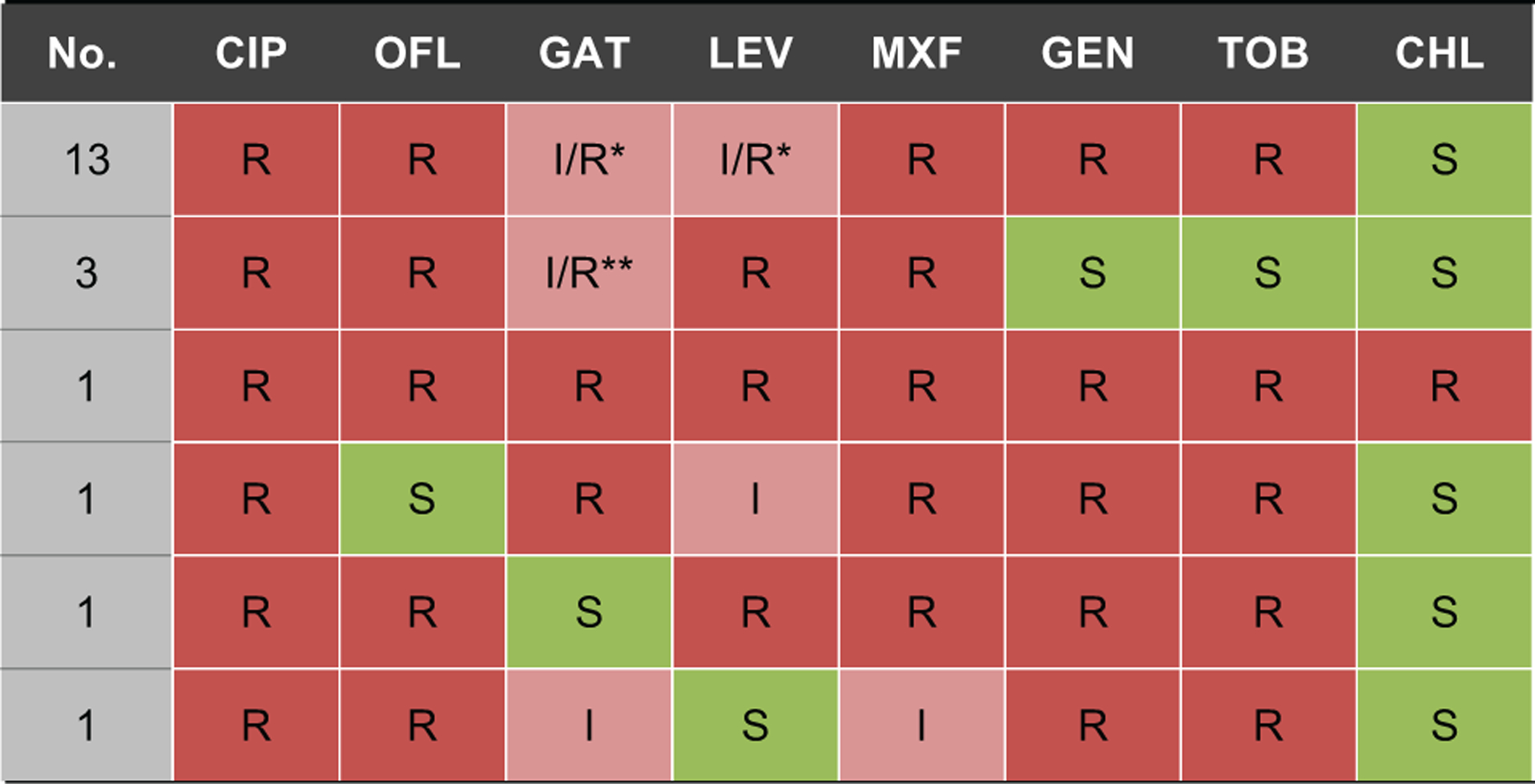
CHL = chloramphenicol; CIP = ciprofloxacin; GAT = gatifloxacin; GEN = gentamicin; I = intermediate susceptibility; LEV = levofloxacin; MOX = moxifloxacin; OFL = ofloxacin; R = resistant; S = susceptible; TOB = tobramycin
*Two isolates had intermediate susceptibility to levofloxacin, 3 isolates had intermediate susceptibility to gatifloxacin, and 2 isolates had intermediate susceptibility to both levofloxacin and gatifloxacin
**One isolate had intermediate susceptibility to gatifloxacin
Methicillin-susceptible S. aureus (MSSA)
Fluoroquinolone non-susceptibility was common in S. aureus, even when only the methicillin-susceptible isolates were analyzed. For example, ofloxacin non-susceptibility was observed in 75 of 177 (42.4%, 95%CI 35.0–50.0%) MSSA isolates. Moreover, the proportion of methicillin-susceptible isolates resistant to most fluoroquinolones increased over the duration of the study (P=0.03 for ciprofloxacin, P=0.002 for ofloxacin, P=0.007 for moxifloxacin, P=0.20 for gatifloxacin, and P=0.42 for levofloxacin; Figure 1). By 2013, fluoroquinolone non-susceptibility was present in 26–66% of methicillin-susceptible S. aureus isolates. The aminoglycocides had variable activity against MSSA over the course of the study, with 43 of 177 (24.3%, 95%CI 18.2–31.3%) isolates resistant to gentamicin and similar numbers resistant to tobramycin. Using breakpoints for viridans group Streptococci, non-susceptibility to vancomycin was not observed in MSSA at any point during the study surveillance period.
Coagulase negative Staphylococci
Antibiotic resistance in coagulase negative Staphylococci was generally similar to S. aureus, with high levels of fluoroquinolone and aminoglycocide non-susceptibility (Figure 1). For example, 70 of 150 (46.7%, 95%CI 38.5–55.0%) isolates were resistant to ofloxacin, and 78 of 151 (51.7%, 95%CI 43.4–59.9%) were resistant to gentamicin. Testing for oxacillin resistance began in 2010; of 66 coagulase negative Staphylococcal isolates tested over the last 4 years of the study, 35 (53.0%, 95%CI 40.3–65.4%) were oxacillin-resistant. Unlike MSSA, no trends in fluoroquinolone non-susceptibility were observed over the duration of the study.
Enterobacteriaceae
Resistance among enteric Gram-negative rods was overall similar to that among P. aeruginosa (Figure 2). For example, non-susceptibility to ofloxacin was documented in 16 of 110 (14.5%, 95%CI 8.5–22.5%) Enterobacteriaceae isolates, and results for the other fluoroquinolones was similar. Gentamicin non-susceptibility was found in 21 of 110 isolates (19.1%, 95%CI 12.2–27.7%). Resistance was similar in other antibiotics with Gram-negative coverage, although amikacin non-susceptibility was slightly less common (12 of 110 isolates resistant; 10.9%, 95%CI 5.8–18.3%; P=0.03 compared with gentamicin non-susceptibility). The proportion of Enterobacteriaceae isolates resistant to the fluoroquinolones appeared to increase slightly over the duration of the study, although this was not statistically significant (e.g., P for trend over time=0.07 for ofloxacin).
DISCUSSION
In South India, the most common causes of bacterial keratitis generally remained susceptible to the fluoroquinolone antibiotics during the 2000s. The exceptions were Nocardia species and Staphylococcus aureus, which demonstrated considerable levels of non-susceptibility to each of the fluoroquinolones. The Gram-positive organisms other than Nocardia that were tested in this study generally remained susceptible to chloramphenicol, and universally remained susceptible to vancomycin, and the Gram-negative organisms generally remained susceptible to the aminoglycosides and ceftazidime. MRSA was seen more commonly over the 12 years of the study, as was fluoroquinolone non-susceptibility among methicillin-susceptible S. aureus. Non-susceptibility to other organisms remained relatively stable over the duration of the study.
Methicillin and fluoroquinolone non-susceptibility each became more common in S. aureus over the duration of this study. These results are consistent with previous publications that have noted an increase in MRSA and an increase in fluoroquinolone resistance among ocular S. aureus species over the 1990s and 2000s.[7–13] These studies reported increases in staphylococcal ciprofloxacin and/or ofloxacin resistance during the 1990s, with up to 40% of isolates demonstrating fluoroquinolone resistance by the early 2000s. Studies from South India have reported similarly high rates of ciprofloxacin resistance in S. aureus during the same time period (resistance in approximately 30% of isolates).[14 15] Previous studies from the United States in the early and mid 2000s have demonstrated fluoroquinolone resistance in approximately 80% of MRSA isolates, but in less than 20% of MSSA isolates.[9 16 17] In contrast, we found a significant increase in fluoroquinolone non-susceptibility even among MSSA in this South Indian study, with 60–66% of isolates resistant to ciprofloxacin, ofloxacin, or moxifloxacin, and 26–46% of isolates resistant to gatifloxacin or levofloxacin in 2013. Aminoglycoside non-susceptibility was also much higher in the MSSA isolates from this South Indian population than has been reported previously in the United States, suggesting variability in the clonal structure of S. aureus populations in different locations.[9 16] The statistically significant increase in fluoroquinolone non-susceptibility among MSSA isolates over the 12 years of the study may be due to increased use of topical fluoroquinolone antibiotics in India, although this theory is speculative. Of note, S. aureus generally remained susceptible to chloramphenicol, suggesting that this drug may be an appropriate choice in South India—especially if vancomycin is not available.
S. pneumoniae isolates displayed more in vitro non-susceptibility to ciprofloxacin than to the other fluoroquinolones. This finding is consistent with some surveillance studies, [18 19] although not others.[16 20] In vitro studies suggest that ciprofloxacin may be more likely to select for resistant pneumococci compared to other fluoroquinolones.[21 22] Compared with the other fluoroquinolones, ciprofloxacin has a lower binding affinity for its enzymatic targets in S. pneumonia (DNA topoisomerase IV and DNA gyrase),[23–25] is more likely to develop resistance through a single mutation,[26] and has a less bulky chemical structure that may allow for easier efflux.[16 27] In addition, ciprofloxacin has been used in India for longer than the other fluoroquinolones, so bacteria have likely been subject to greater selection pressure from ciprofloxacin than from the other fluoroquinolones. Regardless of the mechanism, the results of this study confirm that ciprofloxacin should not be used as the first-line therapy for pneumococcal corneal ulcers in South India, and would not be the best choice for empiric antibiotic therapy when microbiologic testing is inconclusive or not available.
When resistance breakpoints for Staphylococcus were used for Nocardia, none of the Nocardia isolates were found to be resistant to amikacin. In contrast, Nocardia species exhibited high levels of non-susceptibility to the fluoroquinolones, cefazolin, gentamicin, and tobramycin. Amikacin was first reported as a treatment for Nocardia keratitis in 1996.[28] Subsequent studies of Nocardia keratitis isolates, some of which include isolates also described in the current report, have demonstrated near-universal susceptibility to amikacin.[29–31] In the current study, Nocardia isolates remained susceptible to amikacin for the entire study period, further supporting amikacin as the first line treatment for Nocardia keratitis. It is important to note that the CLSI recommends broth microdilution to test Nocardia resistance, whereas in this study we used a disc diffusion assay and applied breakpoints from Staphylococci. However, our results are supported by other studies that have found little in vitro resistance to amikacin when using broth microdilution.[32 33] Because amikacin seems to have similar efficacy as the other aminoglycocides for non-Nocardia infections and superior efficacy for Nocardia corneal ulcers, and because of the relatively high proportion of Nocardia corneal ulcers in South India, amikacin would be a good choice for empiric aminoglycocide therapy in this location.
Although not currently recommended by the CLSI, we performed cefazolin susceptibility testing for Gram-positive organisms since this antibiotic is so commonly used for corneal ulcers. In general, cefazolin resistance was uncommon for most of the organisms included in this report, except for Nocardia species and diphtheroids. The relationship between disc diffusion results and clinical efficacy when an antibiotic is applied topically for corneal ulcers is not clear, especially since the concentration of the antibiotic when administered as a fortified topical antibiotic is so high. Although the cefazolin results in this report must be interpreted with caution, it is promising that so little non-susceptibility was observed for this antibiotic.
A major strength of this study is the large sample size, which allowed assessment of individual bacterial species with relatively high precision. Other strengths include the variety of antibiotics for which susceptibility was tested and the long duration of the study. Moreover, we accounted for autocorrelation between adjacent time points when assessing for temporal trends, which provides a more accurate P-value and has rarely been done in previous studies. There are also several limitations. Most importantly, this is a single-center study, and therefore the resistance patterns cannot necessarily be extrapolated outside of South India. Regardless, the results have important implications given the large population of South India and the high rate of corneal ulcers there. The study is a retrospective analysis of antibiotic susceptibility tests that were done as part of routine clinical practice, and is therefore subject to the biases inherent in such studies. The study includes only in vitro resistance, and like all such studies, defines susceptibility breakpoints according to published standards. These standards are based on the correlation between systemic antibiotic concentrations and clinical response for non-ocular disease, and may be different for topically applied antibiotics, which typically can attain much higher concentrations than systemic administration.[34] Finally, Aravind Eye Hospital is a referral center, so the susceptibility patterns of this study may be different from that of the general population in South India.
In conclusion, this study from South India found that fluoroquinolone antibiotics other than ciprofloxacin generally would offer similar empiric coverage for Gram-positive and Gram-negative organisms as would the aminoglycocides and cefazolin. The proportion of isolates identified as MRSA increased over the latter years of the study but nonetheless remained a minority of the S. aureus that was cultured. Fluoroquinolone non-susceptibility in MSSA increased over the course of the study but the susceptibility of MSSA to chloramphenicol and vancomycin remained high. Culture and susceptibility testing are important in south India, where roughly 15–20% are not susceptible to typically used empiric therapies.
Supplementary Material
Funding
Dr. Keenan was supported by career development awards from the National Eye Institute (K23 EY019071) and Research to Prevent Blindness.
Footnotes
Competing Interests
None of the authors declares a competing interest.
References
- 1.Gonzales CA, Srinivasan M, Whitcher JP, Smolin G. Incidence of corneal ulceration in Madurai district, South India. Ophthalmic epidemiology 1996;3(3):159–66. [DOI] [PubMed] [Google Scholar]
- 2.Upadhyay MP, Karmacharya PC, Koirala S, et al. The Bhaktapur eye study: ocular trauma and antibiotic prophylaxis for the prevention of corneal ulceration in Nepal. The British journal of ophthalmology 2001;85(4):388–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010. Br J Ophthalmol 2012;96(5):614–8 doi: 10.1136/bjophthalmol-2011-300539. [DOI] [PubMed] [Google Scholar]
- 4.Ganguly NK, Arora NK, Chandy SJ, et al. Rationalizing antibiotic use to limit antibiotic resistance in India. Indian J Med Res 2011;134:281–94. [PMC free article] [PubMed] [Google Scholar]
- 5.Kotwani A, Holloway K. Trends in antibiotic use among outpatients in New Delhi, India. BMC Infect Dis 2011;11:99 doi: 10.1186/1471-2334-11-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuper KM, Boles DM, Mohr JF, Wanger A. Antimicrobial susceptibility testing: a primer for clinicians. Pharmacotherapy 2009;29(11):1326–43 doi: 10.1592/phco.29.11.1326. [DOI] [PubMed] [Google Scholar]
- 7.Alexandrakis G, Alfonso EC, Miller D. Shifting trends in bacterial keratitis in south Florida and emerging resistance to fluoroquinolones. Ophthalmology 2000;107(8):1497–502. [DOI] [PubMed] [Google Scholar]
- 8.Goldstein MH, Kowalski RP, Gordon YJ. Emerging fluoroquinolone resistance in bacterial keratitis: a 5-year review. Ophthalmology 1999;106(7):1313–8. [PubMed] [Google Scholar]
- 9.Marangon FB, Miller D, Muallem MS, Romano AC, Alfonso EC. Ciprofloxacin and levofloxacin resistance among methicillin-sensitive Staphylococcus aureus isolates from keratitis and conjunctivitis. Am J Ophthalmol 2004;137(3):453–8 doi: 10.1016/j.ajo.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 10.Fong CF, Hu FR, Tseng CH, Wang IJ, Chen WL, Hou YC. Antibiotic susceptibility of bacterial isolates from bacterial keratitis cases in a university hospital in Taiwan. Am J Ophthalmol 2007;144(5):682–89 doi: 10.1016/j.ajo.2007.06.038. [DOI] [PubMed] [Google Scholar]
- 11.Cavuoto K, Zutshi D, Karp CL, Miller D, Feuer W. Update on bacterial conjunctivitis in South Florida. Ophthalmology 2008;115(1):51–6 doi: 10.1016/j.ophtha.2007.03.076. [DOI] [PubMed] [Google Scholar]
- 12.Chang VS, Dhaliwal DK, Raju L, Kowalski RP. Antibiotic Resistance in the Treatment of Staphylococcus aureus Keratitis: a 20-Year Review. Cornea 2015;34(6):698–703 doi: 10.1097/ICO.0000000000000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hernandez-Camarena JC, Graue-Hernandez EO, Ortiz-Casas M, et al. Trends in Microbiological and Antibiotic Sensitivity Patterns in Infectious Keratitis: 10-Year Experience in Mexico City. Cornea 2015;34(7):778–85 doi: 10.1097/ICO.0000000000000428. [DOI] [PubMed] [Google Scholar]
- 14.Kunimoto DY, Sharma S, Garg P, Rao GN. In vitro susceptibility of bacterial keratitis pathogens to ciprofloxacin. Emerging resistance. Ophthalmology 1999;106(1):80–5 doi: 10.1016/S0161-6420(99)90008-8. [DOI] [PubMed] [Google Scholar]
- 15.Sharma V, Sharma S, Garg P, Rao GN. Clinical resistance of Staphylococcus keratitis to ciprofloxacin monotherapy. Indian J Ophthalmol 2004;52(4):287–92. [PubMed] [Google Scholar]
- 16.Asbell PA, Colby KA, Deng S, et al. Ocular TRUST: nationwide antimicrobial susceptibility patterns in ocular isolates. Am J Ophthalmol 2008;145(6):951–58 doi: 10.1016/j.ajo.2008.01.025. [DOI] [PubMed] [Google Scholar]
- 17.Ni N, Nam EM, Hammersmith KM, et al. Seasonal, geographic, and antimicrobial resistance patterns in microbial keratitis: 4-year experience in eastern Pennsylvania. Cornea 2015;34(3):296–302 doi: 10.1097/ICO.0000000000000352. [DOI] [PubMed] [Google Scholar]
- 18.Parmar P, Salman A, Kalavathy CM, et al. Comparison of topical gatifloxacin 0.3% and ciprofloxacin 0.3% for the treatment of bacterial keratitis. Am J Ophthalmol 2006;141(2):282–86 doi: 10.1016/j.ajo.2005.08.081. [DOI] [PubMed] [Google Scholar]
- 19.Kaliamurthy J, Nelson Jesudasan CA, Geraldine P, Parmar P, Kalavathy CM, Thomas PA. Comparison of in vitro susceptibilities of ocular bacterial isolates to gatifloxacin and other topical antibiotics. Ophthalmic Res 2005;37(3):117–22 doi: 10.1159/000084270. [DOI] [PubMed] [Google Scholar]
- 20.Mather R, Karenchak LM, Romanowski EG, Kowalski RP. Fourth generation fluoroquinolones: new weapons in the arsenal of ophthalmic antibiotics. Am J Ophthalmol 2002;133(4):463–6. [DOI] [PubMed] [Google Scholar]
- 21.Madaras-Kelly KJ, Demasters TA. In vitro characterization of fluoroquinolone concentration/MIC antimicrobial activity and resistance while simulating clinical pharmacokinetics of levofloxacin, ofloxacin, or ciprofloxacin against Streptococcus pneumoniae. Diagn Microbiol Infect Dis 2000;37(4):253–60. [DOI] [PubMed] [Google Scholar]
- 22.Klepser ME, Ernst EJ, Petzold CR, Rhomberg P, Doern GV. Comparative bactericidal activities of ciprofloxacin, clinafloxacin, grepafloxacin, levofloxacin, moxifloxacin, and trovafloxacin against Streptococcus pneumoniae in a dynamic in vitro model. Antimicrob Agents Chemother 2001;45(3):673–8 doi: 10.1128/AAC.45.3.673-678.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pestova E, Millichap JJ, Noskin GA, Peterson LR. Intracellular targets of moxifloxacin: a comparison with other fluoroquinolones. J Antimicrob Chemother 2000;45(5):583–90. [DOI] [PubMed] [Google Scholar]
- 24.Fukuda H, Hiramatsu K. Primary targets of fluoroquinolones in Streptococcus pneumoniae. Antimicrob Agents Chemother 1999;43(2):410–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yague G, Morris JE, Pan XS, Gould KA, Fisher LM. Cleavable-complex formation by wild-type and quinolone-resistant Streptococcus pneumoniae type II topoisomerases mediated by gemifloxacin and other fluoroquinolones. Antimicrob Agents Chemother 2002;46(2):413–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Vecchi E, Nicola L, Ossola F, Drago L. In vitro selection of resistance in Streptococcus pneumoniae at in vivo fluoroquinolone concentrations. J Antimicrob Chemother 2009;63(4):721–7 doi: 10.1093/jac/dkp020. [DOI] [PubMed] [Google Scholar]
- 27.Beyer R, Pestova E, Millichap JJ, Stosor V, Noskin GA, Peterson LR. A convenient assay for estimating the possible involvement of efflux of fluoroquinolones by Streptococcus pneumoniae and Staphylococcus aureus: evidence for diminished moxifloxacin, sparfloxacin, and trovafloxacin efflux. Antimicrob Agents Chemother 2000;44(3):798–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Denk PO, Thanos S, Thiel HJ. Amikacin may be drug of choice in Nocardia keratitis. Br J Ophthalmol 1996;80(10):928–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lalitha P, Tiwari M, Prajna NV, Gilpin C, Prakash K, Srinivasan M. Nocardia keratitis: species, drug sensitivities, and clinical correlation. Cornea 2007;26(3):255–9 doi: 10.1097/ICO.0b013e318033d853. [DOI] [PubMed] [Google Scholar]
- 30.Sridhar MS, Sharma S, Garg P, Rao GN. Treatment and outcome of nocardia keratitis. Cornea 2001;20(5):458–62. [DOI] [PubMed] [Google Scholar]
- 31.Lalitha P, Srinivasan M, Rajaraman R, et al. Nocardia keratitis: clinical course and effect of corticosteroids. Am J Ophthalmol 2012;154(6):934–39 e1 doi: 10.1016/j.ajo.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McTaggart LR, Doucet J, Witkowska M, Richardson SE. Antimicrobial susceptibility among clinical Nocardia species identified by multilocus sequence analysis. Antimicrobial agents and chemotherapy 2015;59(1):269–75 doi: 10.1128/AAC.02770-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Conville PS, Brown-Elliott BA, Wallace RJ Jr., et al. Multisite reproducibility of the broth microdilution method for susceptibility testing of Nocardia species. Journal of clinical microbiology 2012;50(4):1270–80 doi: 10.1128/JCM.00994-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raizman MB, Rubin JM, Graves AL, Rinehart M. Tear concentrations of levofloxacin following topical administration of a single dose of 0.5% levofloxacin ophthalmic solution in healthy volunteers. Clin Ther 2002;24(9):1439–50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


