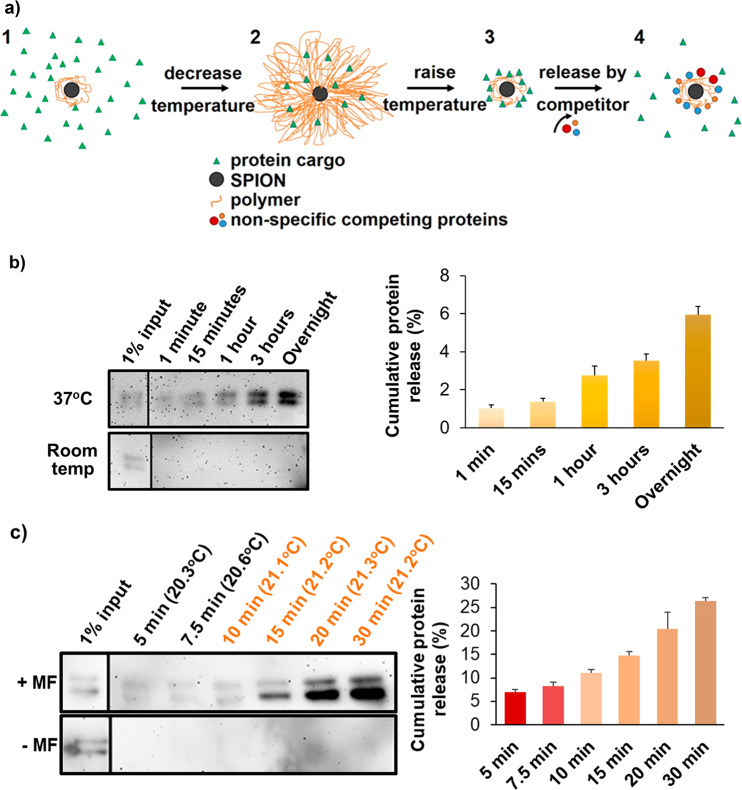
Figure 2.
Protein release from PNIPAM-coated SPIONs. (a) Schematic diagram of protein entrapment and release by PNIPAM-coated SPIONs. (1) 1 μg of protein was mixed with 1 mg of coated SPIONs above the LCST. Polymer chains were collapsed around the nanoparticle core, and agitation was used to avoid particle aggregation. (2) Upon cooling below the LCST, the polymer shell expanded, and some protein molecules were engulfed by the shell; we call this the entrapping state of the coated SPION. (3) Following removal of excess protein by washes in the presence of competing nonspecific proteins, entrapped proteins could be discharged by polymer collapse above the LCST, (4) which permitted nonspecific competitor proteins to replace the weakly bound cargo proteins, thereby releasing them. (b) (Left) Western blot analysis of the apotransferrin collected from the supernatant, following incubation of apotransferrin-loaded PNIPAM-coated nanoparticles (1 mg) in the presence of 10 mg/mL RNase B (the nonspecific competitor) at pH 7.5. SPIONs were briefly collected on one side of the tube with a permanent magnet when the solution was sampled at the indicated time points. (Right) Densitometry of the apotransferrin immunoblot signal used to quantify the amount of apotransferrin released. Error bars denote standard deviation, n = 3. 100% is the amount of protein used for entrapment, given that the amount of protein detected in the washing steps prior to release was negligible. (c) As in part b, but with or without application of an alternating magnetic field (±MF as indicated) turned on constantly for 10 min and then in pulses of 10 s on and 10 s off. The bulk solution temperatures measured during magnetic heating using an IR thermocouple probe are shown in parentheses above each time point. The sample without magnetic heating was maintained at 21 °C. Error bars denote standard deviation, n = 3. Input lanes are from the same blot images but had to be moved because these were not in lanes adjacent to the release samples. Therefore, a divider line was introduced. Note that the double band of apotransferrin represents differently glycosylated forms whose ratios are batch-dependent.