Fig. 1.
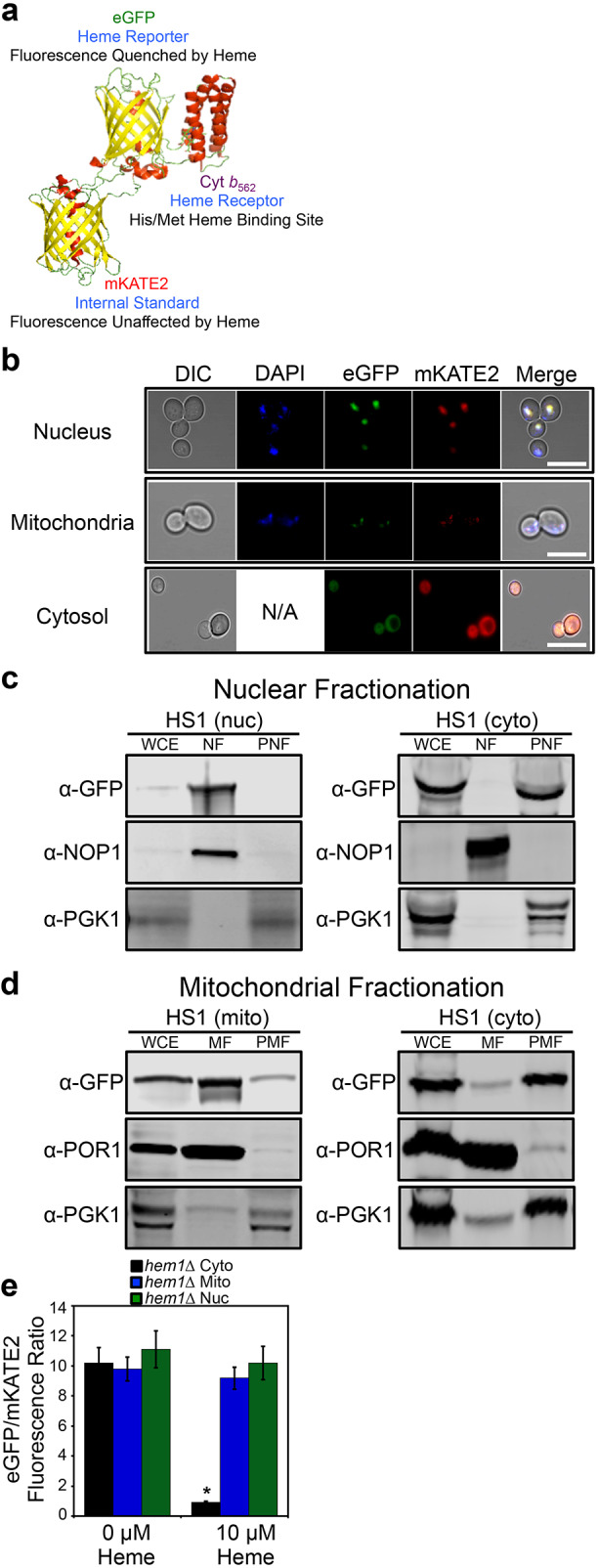
Molecular model and subcellular targeting of the heme sensor HS1. (A) Model of HS1 derived from the X-ray structures of mKATE (PDB: 3BXB) and CG6 (PDB: 3U8P). (B) The subcellular localization of HS1 was assessed by laser-scanning confocal microscopy. Cells expressing nuclear- or mitochondrial-targeted HS1 were stained with DAPI to label nuclei or mitochondria and live cells were imaged at a magnification of 63×. Images shown are representative of n=3 experiments. ‘Merge’ indicates the merged images of fluorescence from the DAPI, EGFP and mKATE2 fluorescence channels. N/A, not applicable. Scale bar: 5 μm. (C,D) Confirmation of mitochondrial (mito), nuclear (nuc), and cytosolic (cyto) HS1 localization as assessed by cell fractionation and immunoblotting. Nuclei (C) and mitochondria (D) were isolated and expression of HS1 was probed using anti-GFP antibodies. The indicated fractions were confirmed by probing the expression of PGK1, NOP1, and POR1, which are cytosolic, nuclear, and mitochondrial marker proteins, respectively. Whole cell extract (WCE), derived from the spheroplast fraction (5% of input loaded), nuclear fraction (NF), mitochondrial fraction (MF), post-mitochondrial fraction (PMF), or post-nuclear fraction (PNF) were electrophoresed on a 14% Tris-glycine SDS–PAGE gel. Blots shown are representative of n=2 experiments. (E) Validation that mitochondrial- and nuclear-targeted HS1 do not respond to cytosolic heme. hem1Δ cells expressing cytosolic, nuclear, or mitochondrial HS1 were permeabilized with digitonin, a plasma-membrane-specific permeabilizing agent, and incubated with the indicated concentration of heme. Fluorimetry data represent mean±s.d. of three biological replicates. *P<0.00001 (one-way ANOVA with Dunnett's post-hoc test).
