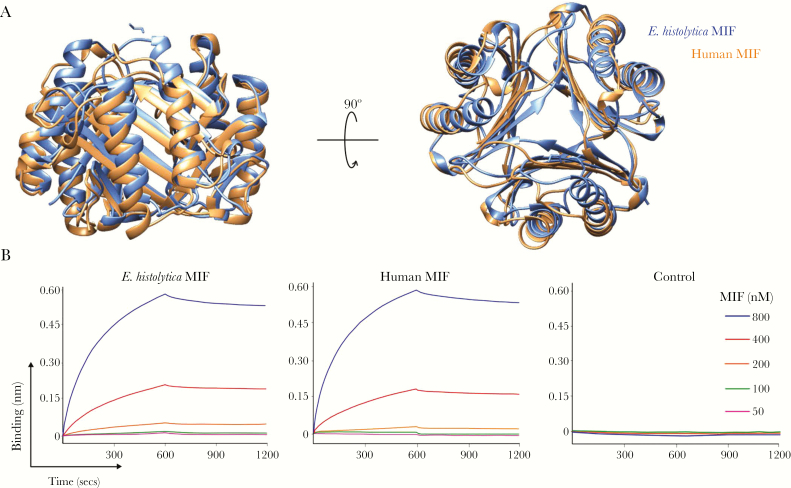
Figure 1.
Characterization of Entamoeba histolytica macrophage migration inhibitory factor (MIF) as a bona fide homolog of human MIF. A, Structural homology between E. histolytica MIF and human MIF. The trimeric form of the human MIF (orange) and E. histolytica MIF (blue) was superimposed as shown in the overlapping backbone ribbon diagram. B, Biolayer interferometry analysis of E. histolytica MIF and human MIF binding to the MIF receptor CD74. Analysis of the representative binding curves (association analysis: 0–600 seconds) and dissociation curves (dissociation analysis: 600–1200 seconds) revealed a dissociation constant (KD) of 1.12 × 10−8 M and 1.82 × 10−8 M for E. histolytica MIF and human MIF, respectively. Biotinylated MBP-CD74 was loaded onto streptavidin biosensors and placed into solutions with MIF (concentration range, 50–800 nM). Biosensors loaded with biotinylated maltose binding protein only were used as a control.