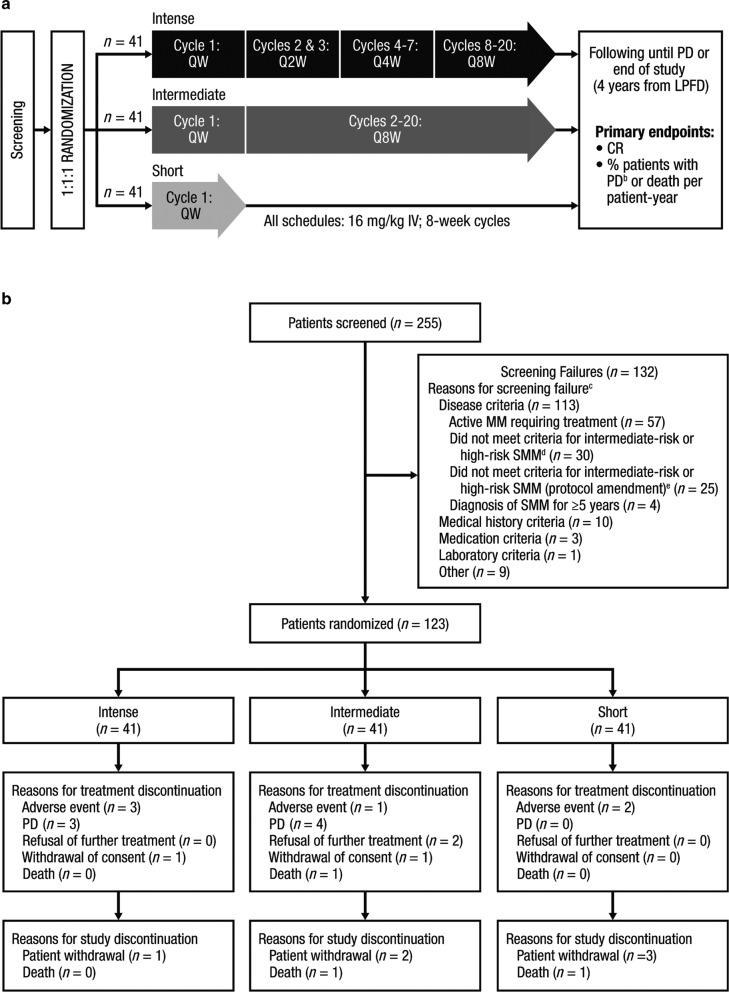
Fig. 1. Study design and patient flow diagram.
a Study design. b Patient flow diagram through the clinical cutoff date.a QW once weekly, Q2W every 2 weeks, Q4W every 4 weeks, Q8W every 8 weeks, IV intravenously, PD progressive disease, LPFD last patient first dose, CR complete response, MM multiple myeloma, SMM smoldering multiple myeloma, IMWG International Myeloma Working Group, FLC free light chain, PC plasma cell. aJune 29, 2018. bPD was defined per the 2014 IMWG criteria for MM [6] plus additional IMWG FLC progression criteria (a ≥25% increase from nadir in the difference between involved and uninvolved FLC levels [absolute increase must be >10 mg/dl]) [21]. cA patient could have multiple reasons for exclusion and therefore be counted in more than one category. dBone marrow PCs ≥10% to <60% plus serum M-protein ≥3 g/dl (IgA ≥2 g/dl), urine M-protein >500 mg/24 h, or abnormal FLC ratio (<0.126 or >8) and serum M-protein <3 g/dl but ≥1 g/dl. eBone marrow PCs ≥10% to <60% plus serum M-protein ≥3 g/dl (IgA ≥2 g/dl), urine M-protein >500 mg/24 h, abnormal FLC ratio (<0.126 or >8) and serum M-protein <3 g/dl but ≥1g/dl, or absolute involved serum FLC ≥100 mg/l with an abnormal FLC ratio (<0.126 or >8, but not ≤0.01 or ≥100).