Abstract
Circular RNAs (circRNAs) are a new class of noncoding single-stranded RNAs that differ from linear microRNAs (miRNAs), since they form covalently closed loop structures without free 3′ poly(A) tails or 5′ caps. circRNAs are the competitive endogenous RNAs (ceRNAs) by binding to miRNA through miRNA response elements (MREs) (i.e., “miRNA sponge”), thereby reducing the quantity of miRNA available to target mRNA, subsequently promoting mRNA stability or protein expression, which involves the initiation and progress of human diseases. Owing to these features of abundance, stability, conservative property, and tissue and stage specificity, widely distributing in the extracellular space and in various bodily fluids, circRNAs can be considered as potential biomarkers for various diseases. Here, we reviewed the promising circRNAs being disease biomarkers, focused on their regulatory function by acting as miRNA sponges, and described their roles in cancer, cardiovascular or neurodegenerative diseases, osteoarthritis, rheumatoid arthritis, diabetes, and other human aging-related diseases, which provide a new direction for pathogenesis, diagnosis, and treatment of human aging-related diseases.
Keywords: circular RNAs, microRNAs, microRNA sponge, diseases, biomarkers
Graphical Abstract
This review highlights our understanding of circular RNAs (circRNAs) being promising disease markers, focusing on their functions as an “miRNA sponge,” which reduces the quantity of miRNAs available to target mRNA, subsequently promoting mRNA stability or protein expression, therefore involved in human aging-related diseases.
Main Text
Circular RNAs (circRNAs) are a new class of noncoding single-stranded RNA,1 characterized by covalently closed loop structures without free 3′ poly(A) tails or 5′ caps. These features differentiate them from linear RNAs,2 long noncoding RNAs (lncRNAs), and microRNAs (miRNAs).3 Although circRNAs were first discovered in the 1990s in viruses, viroids, and tetrahymena,4 little attention has been paid to their function.4,5 At that time, they were considered abnormal products, resulting from splicing errors.3,6 In addition, circRNAs are often found in low abundance, and the traditional methods used to study linear RNAs are not applicable. With recent developments in biochemical-enrichment methods, especially high-throughput RNA sequencing and circRNAs microarray, more than 30,000 circRNAs have been discovered.7 They are widely expressed in yeasts, plants, protists, fruit flies, worms, zebrafish, mice, rats, and humans.8
Compared with the levels of their linear isomers, circRNA expression levels can be increased by 10-fold or more,9 an indication of their potential abundance. Owing to their distinctive structure, they can resist exonuclease activity and are extremely stable.1 The average lifetime of a 3′ → 5′-linked circRNA is 2∼5 times longer than that of a linear mRNA.1 In addition, the expression levels of circRNAs are tissue and stage specific, and a number of highly abundant circRNAs have been found to exist in human peripheral blood (PB),10 indicating that circRNAs can act as biomarkers to screen, diagnose, characterize, and monitor various diseases. circRNAs have many biological functions, including regulating host gene splicing and transcription,11 acting as miRNA12 and protein sponges,8 reacting with proteins,13 and serving as protein-coding circRNAs.14 Many studies15, 16, 17 have indicated that circRNAs contain a large number of miRNA binding sites. As competitive endogenous RNAs (ceRNAs), circRNAs can function as an miRNA sponge and competitively combine with the same miRNAs by miRNA response elements (MREs), removing or reducing the inhibition of genes targeted by the miRNAs and regulating the expression of the target genes.18,19 circRNA not only participates in the proliferation, differentiation, and aging of normal cells but also plays an important role in the pathogenesis of diseases via a large regulatory network of sponge.20,21 Concerning an important pathway, the novel pathological functions of these circRNA-miRNA-mRNA axes in diseases have been expanded over the last few years,22 and circRNA-miRNA-mRNA axes cannot only provide a new direction for pathogenesis, diagnosis, and treatment of diseases but also can act as an advanced molecular technology to simulate or manufacture therapeutic agents, which indicates that this regulatory function of circRNAs, by acting as miRNA sponges, should be a focus of research.
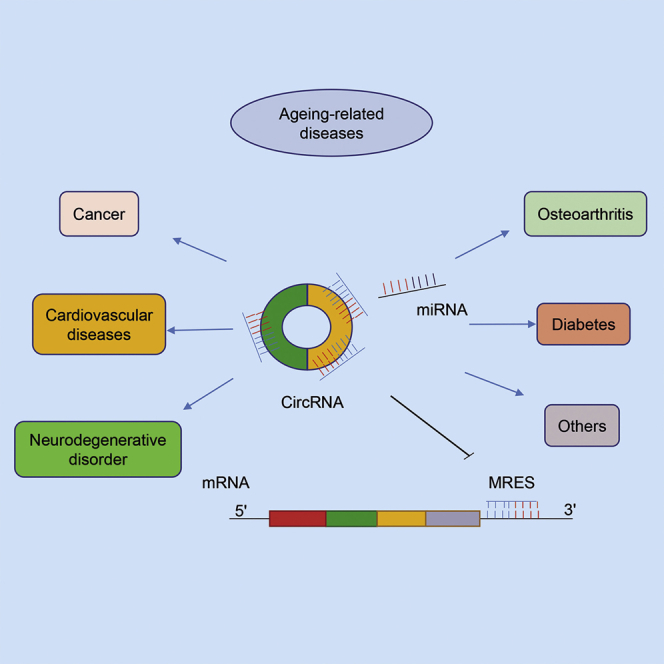
With the gradual aging of the population, the exploration of the biological basis of aging and related molecular mechanisms has become an important topic in modern scientific research.23 Aging can cause the decay of multiple organ functions, leading to the occurrence and development of various aging-related diseases. These include tumors, heart failure, coronary artery disease (CAD), Alzheimer’s disease (AD), osteoporosis, and diabetes. Recently, accumulating evidence has supported the notion that circRNAs are involved in the development of multiple diseases, especially aging-related diseases, such as cancer,24,25 cardiovascular diseases,8 neurological disorders,2 osteoarthritis,26,27 inflammatory diseases,28 and diabetes.29
Here, we highlight recent advances in our understanding of circRNA biogenesis and expression, focusing on the function of miRNA sponges, which serve as biomarkers in these human diseases.
Biogenesis of circRNAs in Eukaryotes
circRNAs are widespread in the eukaryotic genome. In eukaryotes, RNA polymerase II (RNA Pol II) catalyzes the synthesis of precursor mRNAs (pre-mRNAs). The pre-mRNAs undergo spliceosome-mediated splicing to generate linear mRNAs.30 Currently, we do not understand the correlation between RNA circularization and alternative splicing, but several sequence features, such as intron/exon length, hyperdebited RNA, and sequence content, affect the formation of circRNAs.11 It is important to note that the expression levels of circRNAs do not always correlate with those of their linear counterparts, suggesting that the spliceosome can discriminate between linear splicing and RNA circularization, although the mechanism is still unclear.
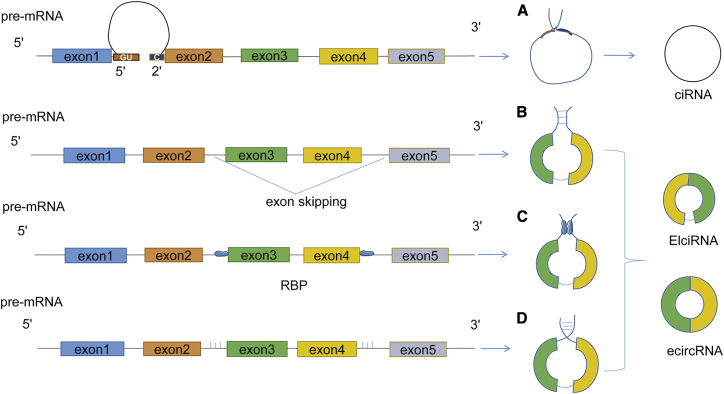
In terms of their genomic origin, circRNAs can be divided into three classes: circular intronic RNA (ciRNA), exonic circRNA (ecircRNA), and exon-intron circRNA (EIciRNA), all of which possess a covalently closed loop structure. These molecules share multiple features, including stability, abundance, conserved properties, and tissue specificity.31 ciRNAs, derived from 2′ → 5′-linked intronic lariats, are generated by canonical splicing32 and are mainly found in the nucleus.33 When pre-mRNAs are spliced, abundant GU elements near the 5′ splice site of the intron and C-rich elements near the branch point escape the debranching enzyme to form the intron lariat.8 ecircRNAs, derived only from exons, represent more than 80% of total circRNAs and are located in the cytoplasm. Most circRNAs are generated from exons (protein-coding genes) or from exons and introns through “back-splicing,” a noncanonical splicing process.32,34 In this process, characterized by exon skipping, with the help of RNA binding proteins (RBPs) 24 or reverse complementary sequences between the flanking introns,31 the downstream splice donor joins the 3′ acceptor splice site of an upstream exon to form the EIciRNA or circRNA24,32 (Figure 1). ecircRNAs, containing only exons, are found mainly in the cytoplasm, whereas EIciRNAs, containing exon and intron regions, are located in the nucleus.
Figure 1.
Biogenesis of Circular RNAs (circRNAs) in Eukaryotes
(A) 2′-5′ ciRNAs are generated when abundant GU elements near the 5′ splice site of the intron and C-rich elements near the branch point escape the debranching enzyme to form an intron lariat. (B–D) EIciRNA and ecircRNA are formed when the downstream splice donor joins the 3′ acceptor splice site of an upstream exon with the help of exon skipping (B), RNA binding proteins (C), or reverse complementary sequences between the flanking introns (D).
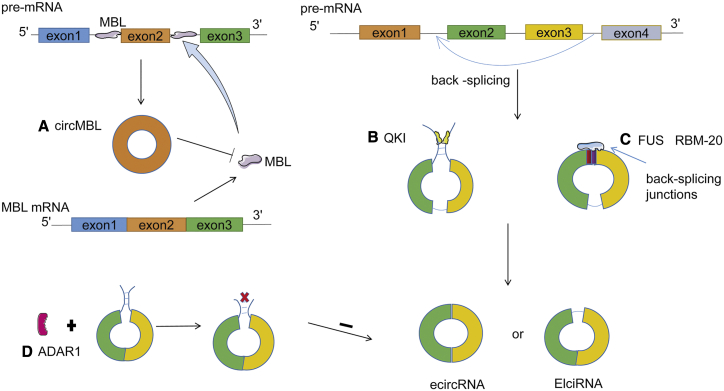
As shown in Figure 2, some RBPs, such as muscleblind (MBL),11 quaking (QKI),35 fused-in-sarcoma (FUS),36 RNA binding motif (RBM)20 protein,37 and double-stranded RNA (dsRNA)-specific adenosine deaminase (ADAR),32 act as splicing factors and regulate circRNA formation. circMbl is formed by back splicing after MBL binds to MBL binding sites in the flanking introns.1 On the other hand, translation of MBL is inhibited by circMbl, which contains the start codon of the main coding sequence,24 indicating that there is a dynamic balance between MBL and circMbl (Figure 2A). QKI is not only a splicing factor that is essential for circulatory and neural development but also a circRNA regulator that plays an important role in the generation of circRNAs.1,35 QKI regulates this process depending on the presence of putative QKI binding sites in the flanking introns of circularized exons. These sites are sufficient to induce circRNA biogenesis, suggesting a simple mechanism that regulates the splicing of circRNA through dimerization of flanking introns (Figure 2B).32 As an RBP, FUS regulates circRNA biogenesis by binding to the introns flanking back-splicing junctions, promoting circRNA production (Figure 2C). Based on this mechanism of splice regulation, studies indicate that FUS participates in the pathogenesis of amyotrophic lateral sclerosis (ALS) and frontotemporal dementia. Similarly, RBM20 also binds to introns flanking back-splicing junctions to regulate the I-band region of the titin transcript, which is known to undergo highly complex alternative splicing and can express approximately 80 circRNAs (Figure 2C).36,37 The quantity of circRNAs is negatively correlated with the expression levels of the RNA-editing enzyme ADAR1, which disrupts RNA-RNA interactions (Figure 2D). Hence, knockdown of ADAR1 significantly enhances circRNA expression in human HEK293 cells.38
Figure 2.
RNA Binding Proteins Regulate the Biogenesis of ecircRNAs and EIciRNAs
(A) circMbl flanking introns have abundant MBL binding sites, MBL promotes the second exon cyclization to form circMbl, and then circMbl and MBL are in dynamic equilibrium. (B) Some introns have QKI binding sites; dimerization through the N-terminal domain of QKI at intron sites can trigger circRNA production. (C) FUS and RBM20 bind to the back-splicing junction to promote circRNA production. (D) ADAR1 negatively regulates circRNA production. ADAR1 converts adenosine to inosine (A-to-I) and breaks RNA-RNA interactions that form during RNA base pairing.
Biological Functions of circRNAs
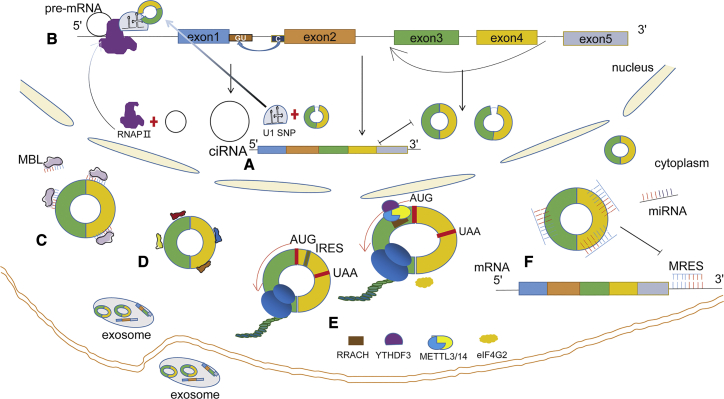
Although the biological functions of circRNAs remain largely unknown, recent studies have demonstrated that circRNAs participate in several steps of gene expression, for example, competing with their linear cognates to regulate promoter activity,27 facilitating protein interactions or encoding functional proteins,13,24 and sponging miRNAs.12
In human cells, circRNAs that are located in the nucleus, such as ciRNAs and EIciRNAs,33 have been found to compete with pre-mRNAs for splicing14 (Figure 3A). It has been noted that knockdown of ciRNAs derived from the introns of ANKRD52, MCM5, and SIRT7, led to decreased expression of their parent genes without affecting nearby genes, suggesting that these ciRNAs act in cis.39 Another study reported that ciRNAs accumulated at the sites of transcription, interacted with the Pol II elongation complex and regulated transcriptional efficiency.40 Similarly, EIciRNAs could combine with U1 small nuclear ribonucleoprotein particle (snRNP) via specific RNA-RNA interactions to form EIciRNA-U1-snRNP complexes and recruit RNA Pol II, promoting RNA Pol II activity in cis and splicing to enhance host gene transcription24,41 (Figure 3B). Suppression of EIciRNA-U1-snRNP interactions blocked the transcription-enhancing effects of EIciRNAs.
Figure 3.
The Functions of Circular RNAs (circRNAs)
(A) circRNAs compete with pre-mRNA linear splicing. (B) circRNAs participate in the process of translation. ciRNAs interact with RNA Pol II (RNAP II), EIciRNAs combine with U1-snRNP via specific RNA-RNA interactions, and EIciRNAs-U1-SNP complexes then recruit RNAP II, promoting RNAP II activity in cis and splicing to enhance host gene transcription. (C) circRNAs can act as protein sponges. Some circRNAs, such as circMbl, have protein binding sites. MBL can combine with circMbl to inhibit the production of circMbl. (D) Some circRNAs can interact with proteins directly. (E) Endogenous circRNAs containing an internal ribosome entry site (IRES) and open reading frame (ORF) can also be translated in a 5′ cap-independent way. m6A-modified circRNAs have the ability to translate proteins. circRNAs can be m6A modified via the METTL3/METTL14 complex, and circRNAs containing a m6A modification site can recruit eIF4G2 protein and other translation initiation factors by recruiting the YTHDF3 protein and then initiate the process of translation. (F) circRNAs can act as miRNA sponges. Natural endogenous circRNAs contain numerous miRNA binding sites; by sequence complementary, they can suppress the activity of miRNAs, promoting mRNA translation.
In addition, circRNAs have been found to sponge or interact with proteins to regulate host gene transcription.8 For example, circMbl originates in the second exon of the splicing factor MBL. In Drosophila, MBL binds to MBL binding sites in the flanking introns to form circMbl.1 At the same time, MBL can combine with circMbl to inhibit the production of circMbl,42 indicating that circRNAs not only modulate alternative splicing but can also act as protein sponges (Figure 3C). Another example is circ-Foxo3 (associated with heart failure), which binds to the age-related proteins ID1 and E2F1, the stress-related protein HIF1a, and FAK in the cytoplasm, inhibiting the entry of these nuclear transcription factors (ID1, E2F1, and HIF1a) into the nucleus, thus inhibiting their function (Figure 3D).13
More surprisingly, some circRNAs that contain an internal ribosome entry site (IRES) and open reading frame (ORF) have been demonstrated to have protein-coding potential in a 5′ cap-independent way,8 thus expanding the eukaryotic proteome. Current evidence indicates that most circRNAs originate from exons and are located in the cytoplasm, which increases the possibility that they may act as protein-coding circRNAs.43 In gliomas, circ-FBXW7 has been found to encode a novel 21-kD protein called FBXW7-185aa through a cross-knot ORF driven by the ribosome entry site. The expression of FBXW7 and FBXW7-185aa was decreased in gliomas, and this had prognostic significance.5 In another study, circRNAs, containing an N6-methyladenosine (m6A) modification site, were found to recruit eIF4G2 protein and other translation initiation factors by recruiting the YTHDF3 protein and then to initiate protein translation via the METTL3/METTL14 complex (Figure 3E).14
More recently, circRNAs have been found to share MREs, allowing them to compete for miRNA binding sites4,44 and function as miRNA sponges, sequestering or reducing the quantity of miRNA available to target mRNA45 and subsequently promoting mRNA stability or protein expression (Figure 3F).24 CDR1as was the first circRNA seminally reported to act as a miRNA sponge, and it contains over 70 conserved miRNA-7 binding sites.1,2 Likewise, cSRY is a circRNA that is specifically expressed in murine testes and harbors 16 binding sites for miRNA (miR)-138.21 Recently, a novel circRNA, termed circHIPK3, originating in exon 2 of the HIPK3 gene, was reported to bind to multiple miRNAs, including miR-124, miR-30a, and miR-558.46, 47, 48
circRNAs Play an Important Role in Aging-Related Diseases by Acting as miRNA Sponges and Can Be Used as Potential Biomarkers
Based upon their biological functions, circRNAs have been found to be involved in the development of human aging-related diseases, such as cancer,25 cardiovascular42 and neurodegenerative32,49,50 disorders, inflammatory27 respiratory diseases,7 and diabetes.29 In addition, since circRNAs are abundant, stable, and conserved and show organ specificity, they have the potential to serve as diagnostic and prognostic biomarkers.31,51, 52, 53 Here, we focus on the regulatory function of miRNA sponges in human aging-related diseases, as well as the roles of circulating circRNAs as potential diagnostic and prognostic biomarkers.31,51,52
circRNAs in Cancer
circRNAs have been well shown to be involved in different aspects of cancer, such as growth, metastasis, stemness, and resistance to therapy.54
For example, hsa_circ_0012673,12 hsa_circ_0000064,55 circPUM1,56 hsa_circ_0007385,57 and circ_000073558 were significantly upregulated in lung carcinoma (LC) tissue when compared with normal adjacent tissue, and there was a correlation between the level of expression of these circRNAs and tumor size.12 In vitro, hsa_circ_0012673 was found to promote the proliferation of LC cells by sponging miR-22.12 Upregulation of circPUM1 has been shown to promote cell proliferation, migration, and invasion in lung adenocarcinoma (LAC)56 and to inhibit apoptosis through a circPUM1-miR-326-cyclin D1/Bcl-2 axis. Through bioinformatics analysis, hsa_circ_0007385 and circ_0000735 were found to sponge miR-18157 and miR-1179/miR-1182,58 respectively, and to promote cell proliferation, migration, and invasion in non-small cell lung cancer (NSCLC). In contrast, downregulated hsa_circ_0043256 in NSCLC59 acted as a miR-1252 sponge and could directly target a vital negative regulator of canonical Wnt signaling, itchy E3 ubiquitin protein ligase (ITCH). Cinnamaldehyde, the primary chemical component of the Chinese traditional herb Cinnamomum cassia and an effective cytotoxic agent against various human cancers, upregulates hsa_circ_0043256 in NSCLC cells, a finding that provides novel insights into lung cancer therapy.59
circRNAs have also been shown to serve as biomarkers and therapeutic targets in hepatocellular carcinoma (HCC). For instance, downregulated circMTO1 was found to be positively correlated with a shortened life expectancy,44 both circMTO1 and hsa_circ_000144560 were found in HCC tissues, and circMTO1 led to a reduction in p21 expression levels by sponging miR-9 and promoting HCC cell proliferation and invasion, suggesting that circMTO1 is a potential prognostic biomarker in HCC.44 On the other hand, it was found that circRNA_100338 regulated metastasis in hepatitis B-related liver cancer by sponging miR-141-3p, and upregulation of circRNA_100338 positively correlated with a low patient survival rate. These findings provide a basis for the possible use of circRNA_100338 as a biomarker to diagnose liver cancer and to understand patient survival rates.61
After comparing the circRNA expression profiles of tumoral versus nontumoral tissues, hsa_circ_0001564,3 hsa_circ_0009910,62 circ_0000502,63 and circ-NT5C264 were found to be overexpressed in osteosarcoma (OS) tissues and cells. Subsequent bioinformatics analyses showed that hsa_circ_0001564 could sponge miR-29c-3p, circ_000050 could sponge miR-1238,3 and hsa_circ_0009910 could sponge miR-449a62 to mediate tumorigenicity. Silencing hsa_circ_0001564,3 hsa_circ_0009910,62 and circ_000050263 significantly suppressed the proliferative activity of OS cells, induced cell-cycle arrest, and triggered OS cell apoptosis. Based on clinicopathological factors and the prognosis of OS patients, researchers found that circ-NT5C2 could act as a molecular biomarker to diagnose and treat OS.64
circRNAs are also aberrantly expressed in gastric adenocarcinoma (GC) tissues. Based on the circRNA expression profile and bioinformatic analysis, circLARP4 might sponge miR-424, which has been shown to inhibit the expression of large tumor suppressor kinase 1 (LATS1) in GC samples and to reduce cell proliferation and invasion, reducing the level of its downstream protein (LATS1).65 Axes, such as circPSMC3-miR-296-5p-PTEN,66 circYAP1-miR-367-5p-p27Kip1,67 and circ-SFMBT2-miR-182-5p-CREB168 have been shown to regulate GC cell growth.57
Abnormal circRNA expression has also been detected in breast cancer (BC) patients, including both upregulated circRNAs associated with the Hippo and Wnt signaling pathways (such as hsa_circ_103110, hsa_circ_104689, and hsa_circ_104821)64 and downregulated circRNAs associated with the RAP1 and Ras signaling pathways (such as hsa_circ_006054, hsa_circ_100219, and hsa_circ_406697).69 Putative interactions between circRNAs and miRNAs69 during the initiation and progression of BC have also been predicted. For example, circRNA-000911, which was downregulated in BC tissues and cell lines, could regulate Notch1 expression by sponging miR-449a, hence regulating nuclear factor κB (NF-κB) signaling.70 circRNA-miRNA axes involving ceRNAs, such as TCDD-hsa_circ_0001098-miR-3942-BARD1,71 hsa_circ_001783-miR-200c-3p,72 hsa_circ_0052112-miR-125a-5p,73 and circ_0005230-miR-618-CBX8, have been found in BC.74 circTADA2A-6E-miR-203a-3p-SOCS375 and circAGFG1-miR-195-5p-CCNE176 have been found in triple-negative breast cancer (TNBC).
Interactions between circRNAs and miRNAs have also been demonstrated in other malignant tumors. For example, the circRNA myosin light chain kinase (MYLK), upregulated in bladder cancer (Bc) tissues and cell lines,77 could act as a miR-29a sponge to increase the expression of VEGFA and activate the vascular endothelial growth factor A (VEGFA)/vascular endothelial growth factor 2 (VEGFR2) signaling pathway and the downstream Ras/extracellular signal-regulated kinase (ERK) signaling pathway, accelerating the progression of Bc. By sponging miR-30c, circPRMT5 could regulate SNAIL1/E-cadherin and induce epithelial-mesenchymal transition, which was positively associated with an advanced clinical stage and worse survival of patients with urothelial carcinoma of the bladder (UCB).78 By comparing the expression levels in colorectal cancer (CRC) tissues with those in adjacent nontumoral tissues or many different types of normal cells, hsa_circ_000753479 was found to be upregulated, and circMTO1 was found to be downregulated in CRC.80 In addition, their abnormal levels of expression were consistent with the tumor stage. hsa_circ_0007534 and circMTO1 are promising as prognostic biomarkers and therapeutic targets in CRC. In addition, the expression of hsa_circ_00016496 has been shown to be reduced in cholangiocarcinoma (CCA), and the expression of circRNA_10029081 has been found to be upregulated in oral squamous cell carcinoma (OSCC).
circRNA expression profiles have also been correlated with gliocytoma. For example, it was shown that circ-TTBK2 sponged miR-217 in a sequence-specific manner, evidenced by the fact that upregulated circ-TTBK2 decreased miR-217 expression, and there was reciprocal negative feedback between them in an argonaute 2-dependent manner. In addition, reintroduction of miR-217 significantly reversed the circ-TTBK2-mediated promotion of glioma progression. Particularly convincing was the demonstration that circ-TTBK2 knockdown combined with miR-217 overexpression led to tumor regression in vivo.82
The roles of these previously mentioned circRNAs and circRNAs/miRNAs in cancer regulation are listed in Table 1.
Table 1.
circRNAs Involved in Cancer
| Cancers | circRNAs | Method | +/− | Axis | Effects | Samples | Ref. |
|---|---|---|---|---|---|---|---|
| LC | hsa_circ_0012673 | circRNA-array | + | hsa_circ_0012673-miR-22-ErbB3 | positive correlates to tumor size; promotes LAC cell proliferation | human LC tissue | 12 |
| LC | hsa_circ_0000064 | N/A | + | promotes cell proliferation and metastasis | human LC tissue and cell lines | 55 | |
| LAC | circPUM1 | N/A | + | circPUM1-miR-326-cyclin D1 and Bcl-2 | promotes LAC cell proliferation, migration, and invasion and inhibits apoptosis | human LAC tissue and cell lines | 56 |
| NSCLC | hsa_circ_0007385 | circRNA-array | + | hsa_circ_0007385-miR-181 | promotes cell proliferation, migration, and invasion | human NSCLC tissues | 57 |
| NSCLC | circ_0000735 | circRNA-array | + | circ_0000735-miR-1179/miR-1182 | associates with more advanced TNM stages; promotes cell proliferation, migration, and invasion | human NSCLC tissues | 58 |
| NSCLC | hsa_circ_0043256 | circRNA-array | − | hsa_circ_0043256-miR-1252-ITCH | promotes cell proliferation and inhibits apoptosis and further directs Wnt/β-catenin pathway | human NSCLC cell lines | 59 |
| HCC | circMTO1 | circRNA-array | − | circMTO1-miR-9-P21 | parallel with the shortening of life; promotes HCC cell proliferation and invasion | human HCC tissues | 44 |
| HCC | hsa_circ_0001445 | N/A | − | promotes HCC cells proliferation, migration, and invasion and inhibits apoptosis | human HCC tissues and cell lines | 60 | |
| HCC | circRNA-100338 | circRNA-array | + | circRNA-100338-miR-141-3p | positively correlates with the cumulative survival rate and cancer metastasis | human HCC tissues | 61 |
| OS | hsa_circ_0001564 | circRNA-array | + | hsa_circ_0001564-miR-29c-3p | silencing of hsa_circ_0001564 significantly suppresses proliferation and promotes apoptosis in HOS and MG-63 cells | human OS tissue | 3 |
| OS | hsa_circ_0009910 | N/A | + | hsa_circ_0009910-miR-449a-IL6R | knockdown of circ_0009910 inhibits OS cell proliferation; induces cell-cycle arrest and apoptosis | human OS tissue and cell lines | 62 |
| OS | circ_0000502 | circRNA-array | + | circ_0000502-miR-1238 | relates to clinical severity; facilitates OS cell proliferation, migration, and invasion; and inhibits apoptosis | human OS tissue and cell lines | 63 |
| OS | circ-NT5C2 | N/A | + | may be a molecular biomarker to diagnose and treat OS | human OS tissues | 64 | |
| GC | RNA_LARP4 | circRNA-array | − | RNA_LARP4-miR-424-5p-LATS1 | associates with pathological stage and unfavorable prognosis of GC patients; promotes GC cell proliferation and invasion | GC tissues | 65 |
| GC | circPSMC3 | circRNA-array | − | circPSMC3-miR-296-5p-PTEN | correlates with higher TNM stage and shorter overall survival; overexpression of circPSMC3 suppresses the proliferation and invasion and inhibits the growth and metastasis of GC cells | human GC tissues, plasma, and cell lines | 66 |
| GC | circYAP1 | N/A | − | circYAP1-miR-367-5p-p27Kip1 | positively correlates survival of GC patients; overexpressed circYAP1 inhibits the proliferation and invasion of GC cells | human GC tissues | 67 |
| GC | circ-SFMBT2 | circRNA-array | + | circ-SFMBT2-miR-182-5p-CREB1 | associates with higher tumor stages of GC; promotes the proliferation of GC cells | human GC tissues and cell lines | 68 |
| BC | hsa_circ_103110, hsa_circ_104689, hsa_circ_104821, hsa_circ_006054, hsa_circ_100219, and hsa_circ_406697 | circRNA-array | hsa_circ_103110, hsa_circ_104689, and hsa_circ_104821(+) associate with Hippo and the Wnt signaling pathway, and hsa_circ_006054, hsa_circ_100219, and hsa_circ_406697(−) associate with RAP1 and the Ras signaling pathway | BC tissues | 69 | ||
| BC | circRNA-000911 | circRNA-array | − | circRNA-000911-miR-449a-Notch1 | overexpression of circRNA-000911 suppresses cell proliferation, migration, and invasion and promotes the apoptosis of BC cells | human BC tissues and cell lines | 70 |
| BC | hsa_circ_0001098 | circRNA-array | + | hsa_circ_0001098-miR-3942-BARD1 | inhibits cell proliferation and promotes cell apoptosis | human BC (treated with TCDD) tissues and cells | 71 |
| BC | hsa_circ_001783 | N/A | + | hsa_circ_001783-miR-200c-3p | correlates with poor clinical outcomes in BC patients; promotes the progress of BC | BC tissues and cell lines | 72 |
| BC | hsa_circ_0052112 | circRNA-array | + | hsa_circ_0052112-miR-125a-5p | promotes cell migration and invasion in BC | MDA-MB-231 cells | 73 |
| BC | circ_0005230 | circRNA-array | + | circ_0005230-miR-618-CBX8 | relates to adverse phenotypes in the patients with BC; as a prognostic predictor in BC patients; promotes BC cell growth and migratory and invasive capacities | BC tissues and cell lines | 74 |
| TNBC | circTADA2As | circRNA-array | − | circTADA2A-6E-miR-203a-3p-SOCS3 | may be prognostic biomarkers for BC | TNBC tissues | 75 |
| TNBC | circAGFG1 | HT RNA-seq | + | circAGFG1-miR-195-5p-CCNE1 | associates with the progression and poor prognosis; promotes TNBC cell proliferation, migration, and invasion and modulates cell cycle and apoptosis; facilitates tumorigenesis, angiogenesis, and metastasis of TNBC cells in vivo | human TNBC tissues and cell lines | 76 |
| Bc | circRNA-MYLK | circRNA-array | + | circRNA-MYLK-miR-29a-VEGFA | relates to the progression of stage and grade of BC; accelerates cell proliferation, migration, and tube formation of HUVEC; rearranges cytoskeleton; promotes EMT through activating VEGFA/VEGFR2 signaling pathway | Bc tissues and cell lines | 77 |
| UCB | circPRMT5 | circRNA-array | + | circPRMT5-miR-30c-SNAIL1/E-cadherin | associates with advanced clinical stage and worse survival; correlates with tumor metastasis | UCB tissues, exosome purification, serum, and urine | 78 |
| CRC | hsa_circ_0007534 | circRNA-array | + | correlates with tumor stage and lymph node metastasis; promotes cell proliferation; and prevents apoptosis | CRC tissues and cell lines | 79 | |
| CRC | circMTO1 | N/A | − | correlates with advanced TNM stage, lymph node metastasis, and poor overall survival; promotes cell proliferation and invasion | CRC tissues and cell lines | 80 | |
| CCA | hsa_circ_0001649 | N/A | − | associates with tumors size and differentiation grade; promotes cell proliferation, migration, and invasion; inhibits apoptosis | CCA tissues and cell lines | 6 | |
| OSCC | circRNA_100290 | circRNA-array | + | circRNA_10 0290-miR-29 family-CDK6 | promotes cell proliferation | OSCC tissues and cell lines | 81 |
| Gliocytoma | circ-TTBK2 | N/A | + | circ-TTBK2-miR-217-HNF1β/Derlin-1 | promotes cell proliferation, migration, and invasion, whereas inhibits apoptosis | Gliocytoma tissues and cell lines | 82 |
LC, lung carcinoma; LAC, lung adenocarcinoma; NSCLC, non-small cell lung cancer; HCC, hepatocellular carcinoma; OS, osteosarcoma; GC, gastric cancer; EMT, epithelial-mesenchymal transition; BC, breast cancer; TNBC, triple-negative breast cancer; Bc, bladder cancer; UCB, urothelial carcinoma of the bladder; CRC, colorectal cancer; CCA, cholangiocarcinoma; OSCC, oral squamous cell carcinoma; HT RNA-seq, high-throughput RNA sequencing; circRNA-array, circRNA microarray; N/A, not available; +, upregulate; −, downregulate; Ref., references; HUVEC, human umbilical vein endothelial cell; CDK6, cyclin-dependent kinase 6; HNF1β, hepatocyte nuclear factor 1beta.
circRNAs in Cardiovascular Diseases
A comprehensive understanding of the role of circRNAs in the pathogenesis of cardiovascular diseases may permit the identification of biomarkers and therapeutic targets for CAD,83 heart failure,84 and myocardial infarction.85
An in-depth investigation of the mechanisms has suggested that the occurrence and development of CAD might be related to the joint regulation of circRNAs and miRNAs.86 It has been shown that circNCX1, which acts as a sponge of miR-133a-3p, is significantly increased in reactive oxygen species (ROS)-producing cardiomyocytes and promotes cardiomyocyte apoptosis by targeting CDIP1.87 In terms of myocardial fibrosis (MF), secondary to myocardial ischemia and hypoxia, caused by moderate/severe coronary atherosclerotic stenosis, circRNA_01056788 and circRNA_00020331 were shown to be significantly upregulated in a model of MF in mice. Through bioinformatics analysis, it was found that circRNA_010567 could sponge miR-141 at more than one binding site, and the 3′ UTR of transforming growth factor β1 (TGF-β1) was highly correlated with miR-141.88 This was verified by the fact that overexpression of circRNA_010567 promoted the expression of TGF-β1 and promoted the expression of the fibrosis-related proteins collagen type I (Col I), Col III, and α-smooth muscle actin (α-SMA).88 circRNA_000203 was found to bind to miR-26b-5p, which bound to the 3′ UTRs of collagen type I α 2 chain (Col1a2) and connective tissue growth factor (CTGF), and this was verified by the fact that overexpression of circRNA_000203 directly promoted the expression of Col1a2 and CTGF.31 However, the correlation between circRNA_010567 and circRNA_000203 in the pathological process of MF is still unclear.
Cardiac hypertrophy (CH) is associated with a significantly increased risk of heart failure (HF), one of the leading causes of death in the world.84 A recent study showed that the expression of mm9-circ-012559, named heart-related circRNA (HRCR), was significantly reduced in an isoproterenol- or aortic arch constriction-induced model of CH in mice, and bioinformatics analysis revealed that HRCR contains six binding sites for miR-223, which targets the 3′ UTR of ARC (apoptosis repressor with CARD domain) to promote CH;84 these findings indicate that mm9-circ-012559 could be a therapeutic target in CH.
Acute myocardial infarction (AMI) has a 30-day mortality rate approaching 10% in most European and North American countries.85 circRNA_081881 was found to be significantly downregulated in the plasma of AMI patients, and western blot analysis showed that the expression of peroxisome proliferator-activated receptor gamma (PPARγ) correlated with circRNA_081881 levels. Additional experiments confirmed that circRNA_081881 sponged miR-548 to regulate the expression of PPARγ in AMI, indicating that it was a potential target for the diagnosis and treatment of AMI.89
The roles of these above-mentioned circRNAs and circRNAs/miRNAs in cardiovascular disease regulation are listed in Table 2.
Table 2.
circRNAs Involved in Cardiovascular Diseases
| Diseases | circRNAs | Method | +/− | Axis | Effects | Samples | Ref. |
|---|---|---|---|---|---|---|---|
| IMI | circNCX1 | Sanger sequencing | + | circNCX1-miR-133a-3p-CDIP1 | promotes cardiomyocyte apoptosis | fetal cardiomyocyte-derived H9c2 cell | 87 |
| MF | circRNA_010567 | circRNA-array | + | circRNA_010567-miR-141-TGF-β1 | promotes Col I, Col III, and α-SMA expression | diabetic mice myocardium and CFs treated with Ang II | 88 |
| MF | circRNA_000203 | circRNA-array | + | circRNA_000203-miR-26b-5p-Col1a2 and CTGF | promotes the expression of Col1a2 and CTGF and promotes myocardial fibrosis | myocardium of the db/db mice | 31 |
| CH and HF | HRCR/(mm9-circ-012559) | N/A | − | HRCR-miR-223-ARC | overexpression of HRCR can decrease cardiomyocyte size, WH/WB, cardiac stress gene expression, and interstitial fibrosis | mouse heart | 84 |
| AMI | circRNA_081881 | circRNA-array | − | circRNA_081881-miR-548-PPARγ | as a potential target for the diagnosis and treatment of AMI | plasma of AMI patients | 89 |
IMI, ischemic myocardial injury; MF, myocardial fibrosis; CFs, mouse cardiac fibroblasts; CH and HF, cardiac hypertrophy and heart failure; WH/WB, heart weight to body weight ratio; AMI, acute myocardial infarction; circRNA-array, circRNA microarray; N/A, not available; +, upregulate; −, downregulate.
circRNAs in Neurodegenerative Diseases
Certain circRNAs have been found to be specifically expressed in the mammalian central nervous system,38 suggesting that they play tissue-specific physiological and pathological roles and may also serve as potential diagnostic biomarkers or therapeutic targets in diseases that affect this organ system.
For example, in rats with sciatic nerve injury (SNI), the miR-34 family connected circRNAs to LC3-II/p62, as evidenced by the fact that circRNA_2837 sponged miR-34 family members and induced autophagy, which in turn, protected neurons from damage.90 In HT22 cells subjected to hypoxia/reoxidation, mmu-circRNA-015947 was upregulated and sponged certain miRNAs associated with neural damage (such as mmu-miR-188-3p, mmu-miR-329-5p, mmu-miR-3057-3p, mmu-miR-5098, and mmu-miR-683), regulating their target gene expression. Based on Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis, mmu-circRNA-015947 has been shown to be associated with pathological processes, such as apoptosis, metabolism, and the immune response during cerebral ischemia-reperfusion.91
circRNAs tend to accumulate during the normal brain aging process and thus, may correlate with susceptibility to aging-related neurodegenerative diseases, such as AD. The role of circRNAs in AD has gradually been recognized.92 For example, evolutionarily conserved miRNA-7 was abundant in the human brain, and its levels correlated with ciRS-7 (also known as CDR1as), a circRNA specific for miRNA-7 and containing multiple tandem anti-miRNA-7 sequences; in addition, ciRS-7 acts as an endogenous anticomplementary miRNA “sponge” to adsorb and quench normal miRNA-7 activity.93 Downregulation of ciRS-7 and ciRS-7 “sponging activity” increased miRNA-7 levels in AD-affected brain cells, ultimately contributing to the downregulation of miRNA-7-sensitive mRNA targets.94 Deficits in other circRNA-mediated “miRNA-sponging systems” and the consequent upregulation of certain endogenous miRNAs may explain the generalized and progressive downregulation of gene expression that is characteristically seen in sporadic AD.95, 96, 97
Parkinson’s disease (PD) is another aging-related neurodegenerative disorder and mainly affects substantia nigra neurons in the brain, causing a common type of dyskinesia.98 It has been reported that miR-7 can inhibit the expression of α-synuclein, a crucial constituent of Lewy bodies in the PD brain, and protect against oxidative stress and the neuronal death induced by 1-methyl-4-phenylpyridinium (MPP+) by targeting the NF-κB signaling pathway.99, 100, 101 In addition, another report indicated that circDLGAP4 was decreased in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced PD mouse model and MPP+-induced PD cell models. Bioinformatics studies suggested that circDLGAP4 acted as a miR-134-5p sponge to regulate CREB signaling and influence the expression of CREB target genes, including brain-derived neurotrophic factor (BDNF), Bcl-2, and peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α), in SH-SY5Y and MN9D cells.102
circRNAs in Osteoarthritis
Osteoarthritis (OA) is a common joint disease characterized by degeneration of articular cartilage.24 In the interleukin (IL)-1β-induced model of arthritis, 255 circRNAs were found to be abnormally expressed, 119 of which were significantly upregulated and 136 of which were downregulated. Gene Ontology (GO) and KEGG pathway analyses revealed that the target genes of these circRNAs were involved in cell metabolism, regulation, and apoptosis. Investigators identified and validated two differentially expressed circRNAs (mmu-circRNA-30365 and mmu-circRNA-36866) that were predicted to function as miRNA sponges. This suggests that circRNAs represent novel molecular targets in OA.24 In another IL-1β-induced model of arthritis, researchers identified a circRNA (circRNA_Atp9b) that was significantly upregulated in chondrocytes and regulated the expression of downstream genes by targeting miR-138-5p, which modulated extracellular matrix (ECM) metabolism and inflammation. This further confirmed that circRNAs can be used as therapeutic targets in OA.27 One study investigated the expression of circRNAs in different regions affected by OA and identified a mechanical stress-related circRNA (circRNA-MSR) that participated in the degradation of chondrocytes by targeting miR-875 to regulate tumor necrosis factor α (TNF-α) expression.103 Additional studies of the process of cartilage degradation have determined that increased levels of IL-1 and TNF-α upregulate ECM-associated circRNA (circRNA-CER). circRNA-CER competed with miR-136 for MMP-13 RNA.104
circRNAs in Diabetes
Progress has been made in exploring the potential role of noncoding RNAs (ncRNAs) in the regulation of gene networks involved in metabolic diseases. Recently, it was demonstrated that circHIPK3 mediated retinal vascular dysfunction in diabetes mellitus (DM) by sponging miR-30a to increase the expression of its target genes.105 Finally, expression of circ_010567 was increased in the myocardium and cardiac fibroblasts of diabetic mice and acting through a mechanism involving miR-141 sponging, led to upregulation of TGF-β1 expression.88
circRNAs in Other Diseases
In the lipopolysaccharide (LPS)-induced model of respiratory distress syndrome, five differentially expressed circRNAs were selected and validated based on high-throughput detection of circRNAs: mmu-circRNA_19423, rno_circRNA_010489, rno_circRNA_011426, and mmu-circRNA_30664 were significantly upregulated, whereas rno_circRNA_005564 was significantly downregulated.106 This result indicates that these circRNAs could potentially be used to diagnose respiratory distress syndrome.
The roles of the previously mentioned circRNAs and circRNAs/miRNAs in the regulation of other diseases are listed in Table 3.
Table 3.
circRNAs Involved in Other Diseases
| Diseases | circRNAs | Method | +/− | Axis | Effects | Samples | Ref. |
|---|---|---|---|---|---|---|---|
| SNI | circRNA.2837 | HT RNA-seq | − | circRNA.2837-miR-34 family-LC3-II/p62 | protects neurons against injury by inducing autophagy | ISN of rats | 90 |
| NI | mmu-circRNA-015947 | circRNA-array | + | mmu-circRNA-015947-mmu-miR-(188-3p, 329-5p, 3057-3p, 5098, 683) | may be involved in the process of IRI | HT22 cells with OGD/R | 91 |
| AD | ciRS-7 | N/A | − | ciRS-7-miRNA-7-UBE2A | regulates the downregulation of UBE2A expression | human AD brain tissues | 93,94 |
| PD | circDLGAP4 | N/A | − | circDLGAP4-miR-134-5p-CREB signaling | inhibits viability, induces apoptosis, increases mitochondrial damage, attenuates autophagy, and thereby increases the neurotoxic effects | MPTP-induced PD mouse model and MPP+-induced PD cell models |
102 |
| OA | mmu-circRNA-30365; mmu-circRNA-36866 | HT RNA-seq | new molecular targets for treatment of OA | IL-1β-induced MACs | 24 | ||
| OA | circRNA_Atp9b | N/A | + | circRNA_Atp9b-miR-138-5p-type II collagen/MMP13/IL-6 and COX-2 |
knockdown of circRNA_Atp9b increases the synthesis of type II collagen, reduces the expression of MMP13, IL-6, and COX-2 | IL-1β-induced MACs | 27 |
| OA | circRNA-MSR | circRNA-array | + | circRNA-MSR-miR-875-TNF-α | silencing of circRNA-MSR will downregulated TNF-α and increase ECM formation | knee joint tissues of patients | 103 |
| OA | circRNA-CER | circRNA-array | + | circRNA-CER-miR-136-MMP13 | silencing of circRNA-CER will downregulate MMP13 expression and increase ECM formation | knee joint tissues of OA patients | 104 |
| DM | circHIPK3 | N/A | + | circHIPK3-miR-30a-VEGFC/Wnt2/FZD4 | silencing circHIPK3 can alleviate retinal vascular dysfunction | human and mouse retinal endothelial cells | 105 |
SNI, sciatic nerve injury; ISN, injured sciatic nerve; NI, neuron injury; IRI, cerebral ischemia-reperfusion injury; OGD/R, oxygen-glucose deprivation/reoxygenation; AD, Alzheimer’s disease; PD, Parkinson’s disease; OA, osteoarthritis; MACs, mouse articular chondrocytes; ECM, extracellular matrix; RA, rheumatoid arthritis; T2DM, type 2 diabetes mellitus; DM, diabetes mellitus; HT RNA-seq, high-throughput RNA sequencing; circRNA-array: circRNA microarray; N/A, not available; +, upregulate; −, downregulate; COX-2, cyclooxygenase 2.
Circulating circRNAs Act as Potential Biomarkers for Diseases
Owing to their covalently closed loop structures, circRNAs can resist degradation by RNases, making them highly stable in plasma, serum, or other biofluids.107 With the development of high-throughput RNA sequencing, several hundred to thousands circRNAs with tissue- and stage-specific characteristics have been identified, and these circRNAs are found in higher levels in PB than intracellular fractions of relevant solid tissues;10 as such, the above-mentioned circulating circRNAs have potential to act as biomarkers to screen, diagnose, characterize, and monitor diseases, such as cancers, cardiovascular diseases, inflammatory respiratory diseases, diabetes, and others.
circRNA expression profiles between GC patients and healthy individuals were compared, and circ-KIAA1244 was found to be expressed at lower levels in GC plasma than in plasma from healthy individuals and was negatively correlated with tumor node metastasis (TNM) stage, lymphatic metastasis, and a shorter overall survival time of GC patients, which indicated that circ-KIAA1244 could serve as a novel circulating biomarker for detecting GC.108 In addition, hsa_circ_0000520109 and circ_0009910110 were anomalously expressed in GC plasma and had the potential to be used as prognostic biomarkers. However, the correlation among circ-KIAA1244, hsa_circ_0000520, and circ_0009910 still needs to be researched. Another study indicated the potential of hsa_circ_0005962 and hsa_circ_0086414 as circulating circRNAs to diagnose LAC.111 Circulating exosomal hsa_circ-0004771 was significantly upregulated in CRC patients and served as a novel potential diagnostic biomarker of CRC.112 Based on receiver operating characteristic (ROC) curve analysis and its correlation with clinical characteristics, such as histological grade, TNM stage, and distant metastasis, hsa_circ_0001785, as a circulating circRNA in the PB of BC patients, was selected as the best diagnostic marker for BC.113 In UCB, circPRMT5 was positively associated with advanced clinical stage and poor survival of patients.78
Early diagnosis of congenital heart disease (CHD) could improve disease prognosis in childhood and reduce mortality.114 One study indicated that three circRNAs (hsa_circRNA_004183, hsa_circRNA_079265, and hsa_circRNA_105039) were significantly downregulated in the plasma of children with CHD, suggesting that these circRNAs may serve as novel plasma biomarkers for the diagnosis of CHD in children.114 Based on the differential expression profiles in PB determined by RNA microarray and ROC curve analysis, hsa_circ_0124644 has been postulated as a diagnostic biomarker for CAD83. In the PB of patients with AMI, the expression of myocardial infarction-mediated circRNA (MICRA) was downregulated. Univariate and multivariate logistic regression analyses showed that MICRA was a powerful predictor of left ventricular dysfunction—the lower the MICRA levels were, the higher the risk of left ventricular dysfunction.115 Investigators determined that MICRA expression levels after AMI were associated with three separate risk stratification levels (3EF), further demonstrating that MICRA could be used as a circulating biomarker for risk prediction after AMI.85
Rheumatoid arthritis (RA) is a chronic synovitis of unknown etiology that can lead to joint deformities and loss of function.23,116 By comparing circRNA expression in peripheral blood mononuclear cells (PBMCs) of RA patients with healthy volunteers, the following circRNAs were identified: hsa_circRNA_104194, hsa_circRNA_104593, hsa_circRNA_103334, hsa_circRNA_101407, and hsa_circRNA_102594, which suggested that these circRNAs could potentially be used as circulating circRNAs to diagnose and treat RA.28 Other studies identified and validated five differentially expressed circRNAs (circRNAs 092516, 003524, 103047, 104871, and 101873) with high area under the curve (AUC) values in PBMCs of RA patients, suggesting that these circRNAs could serve as diagnostic biomarkers for RA.103 In addition, hsa_circ_0044235 was decreased significantly in the PB of RA patients, and ROC curve analysis suggested that hsa_circ_0044235 had significant value in the diagnosis of RA.117
hsa_circ_0054633 was abnormally expressed in the PB of diabetic patients, and two cohort tests determined that hsa_circ_0054633 showed a good AUC. At the same time, GO analysis showed that hsa_circ_0054633 participated not only in biological processes but also in molecular metabolic processes. The above results suggested that hsa_circ_0054633 could be used as a diagnostic marker for prediabetes and type 2 diabetes.29
Tuberculosis (TB) is an infectious disease caused by Mycobacterium tuberculosis (Mtb) and a leading cause of global morbidity and mortality.45 Identification of early diagnostic biomarkers for TB would benefit the control of infection. By analyzing the expression profiles of circRNAs in PBMCs of TB patients, researchers found that hsa_circRNA_001937 was significantly upregulated in pulmonary tuberculosis compared with other lung diseases, such as pneumonia, lung cancer, or chronic obstructive pulmonary disease (COPD). Furthermore, with recovery from TB, the expression of hsa_circRNA_001937 was reduced. Likewise, analysis of the ROC curve of hsa_circRNA_001937 suggested that its AUC was high, indicating that this circRNA could be used as a circulating biomarker for the diagnosis and treatment of TB.45
Based on the special characteristics of circRNAs and their roles, detailed in the above discussions, in various aging-related diseases, circulating circRNA-based diagnostic and prognostic tools for diseases could become a reality. The roles of the previously mentioned circulating circRNAs as potential biomarkers for aging-related diseases are listed in Table 4.
Table 4.
Circulating circRNA Acted as a Potential Biomarker for Diseases
| Diseases | circRNAs | Method | + /− | Samples | Ref. |
|---|---|---|---|---|---|
| GC | circ-KIAA1244 | circRNA-array | − | plasma of GC patients | 108 |
| GC | hsa_circ_0000520 | circRNA-array | − | plasma of GC patients | 109 |
| GC | circ_0009910 | N/A | + | plasma of GC patients | 110 |
| LAC | hsa_circ_0005962; hsa_circ_0086414 | N/A | plasma of LAC patients | 111 | |
| CRC | hsa_circ-0004771 | N/A | + | exosomes from serum of CRC patients | 112 |
| BC | hsa_circ_0001785 | circRNA-array | PB of BC patients | 113 | |
| UCB | circPRMT5 | circRNA-array | + | UCB tissues, exosome purification, serum, and urine | 78 |
| CHD | hsa_circRNA_004183, hsa_circRNA_079265, and hsa_circRNA_105039 | circRNA-array | − | plasma of children with CHD | 114 |
| CAD | hsa_circ_0124644 | circRNA-array | + | PB of CAD patients | 83 |
| AMI | MICRA | N/A | − | PB of AMI patients | 85,115 |
| RA | hsa_circRNA (104194, 104593, 103334, 101407, and 102594) | circRNA-array | PBMC of RA patients | 28 | |
| RA | circRNAs (092516, 003524, 103047, 104871, and 101873) | circRNA-array | + | PBMC of RA patients | 103 |
| RA | hsa_circ_0044235 | circRNA-array | − | PB of RA patients | 117 |
| T2DM | hsa_circ_0054633 | circRNA-array | PB of T2DM patients | 29 | |
| TB | hsa_circRNA_001937 | circRNA-array | + | PBMC of TB patients | 45 |
GC, gastric adenocarcinoma; LAC, lung adenocarcinoma; CRC, colorectal cancer; BC, breast cancer; UCB, urothelial carcinoma of the bladder; CHD, congenital heart disease; CAD, coronary artery disease; AMI, acute myocardial infarction; RA, rheumatoid arthritis; T2DM, type 2 diabetes mellitus; TB, tuberculosis; PB, peripheral blood; PBMC, peripheral blood mononuclear cell; circRNA-array, circRNA microarray; N/A, not available; +, upregulate; −, downregulate.
Conclusions
Elucidation of the function of circRNAs allows us to understand their role in health and disease. Due to their structural stability and presence in exosomes, circRNAs can act in an autocrine, a paracrine, and possibly even an endocrine manner. In addition, the fact that circRNAs are widely distributed not only within cells but also in the extracellular space and in various bodily fluids makes them ideal biomarker candidates for various human diseases. As with any emerging field, caution is advised when interpreting the new findings. To date, studies are merely descriptive in nature. More in-depth analysis of the function and regulation of identified circRNAs is needed to understand their mechanism of action. These RNA molecules hold great promise as disease biomarkers because of their high resistance to exonuclease activity and because they might even be more highly expressed than their linear counterparts. Their putative role as miRNA sponges makes them interesting targets for future investigations. In the future, circRNA-based therapies could become a reality. However, the concept of circRNA-based therapies depends on their stability, differential expression in distinct organs, and specificity for a certain disease. As is the case for miRNAs, the likelihood of triggering significant and undesired off-target effects can greatly limit the use of circRNAs as therapeutics. Hence, the more specific circRNAs are to a specific organ or disease, the less likely they will be to have off-target effects. Elucidation of the role of specific circRNAs is a prerequisite for targeted RNA-based therapies for specific diseases. Alternatively, tissue/cell specificity might be enhanced by coupling ncRNAs to tissue-specific antibodies and/or peptides, thereby reducing off-target effects.
Limitations
circRNAs are being increasingly studied in the RNA field. However, there are still some issues that need to be resolved. How are circRNAs degraded? What other biological functions do circRNAs have in health and disease? How can we standardize the naming of circRNAs? Finally, in a given disease, some circRNAs are upregulated, whereas others are simultaneously downregulated. What are the regulatory relationships between upregulated and downregulated circRNAs? These issues need to be explored in more detail.
Author Contributions
Conceptualization, L.S.; Writing – Original Draft preparation, L.S. and S.R.; Writing – Review & Editing, L.S.; Funding Acquisition, L.S.; Software, H.Y.; Visualization, J.W.; Writing – Review, P.L.; Reviewing, G.D.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
We acknowledge funding support from the following: National Natural Science Foundation of China (grant number 81670456), Major Science and Technology Project for “Significant New Drugs Creation” (2018ZX09711001-003-011 and 2018ZX09711001-012), Innovation Fund for Medical Sciences (grant number 2017-I2M-1-011), and Beijing Municipal Natural Science Foundation (grant number 7162132).
Contributor Information
Lan Sun, Email: sunhanxing2005@imm.ac.cn.
Guanhua Du, Email: dugh@imm.ac.cn.
References
- 1.Holdt L.M., Kohlmaier A., Teupser D. Molecular roles and function of circular RNAs in eukaryotic cells. Cell. Mol. Life Sci. 2018;75:1071–1098. doi: 10.1007/s00018-017-2688-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen Y., Li C., Tan C., Liu X. Circular RNAs: a new frontier in the study of human diseases. J. Med. Genet. 2016;53:359–365. doi: 10.1136/jmedgenet-2016-103758. [DOI] [PubMed] [Google Scholar]
- 3.Song Y.Z., Li J.F. Circular RNA hsa_circ_0001564 regulates osteosarcoma proliferation and apoptosis by acting miRNA sponge. Biochem. Biophys. Res. Commun. 2018;495:2369–2375. doi: 10.1016/j.bbrc.2017.12.050. [DOI] [PubMed] [Google Scholar]
- 4.Hu J., Li P., Song Y., Ge Y.X., Meng X.M., Huang C., Li J., Xu T. Progress and prospects of circular RNAs in Hepatocellular carcinoma: Novel insights into their function. J. Cell. Physiol. 2018;233:4408–4422. doi: 10.1002/jcp.26154. [DOI] [PubMed] [Google Scholar]
- 5.Yang Y., Gao X., Zhang M., Yan S., Sun C., Xiao F., Huang N., Yang X., Zhao K., Zhou H. Novel Role of FBXW7 Circular RNA in Repressing Glioma Tumorigenesis. J. Natl. Cancer Inst. 2018;110:304–315. doi: 10.1093/jnci/djx166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu Y., Yao Y., Zhong X., Leng K., Qin W., Qu L., Cui Y., Jiang X. Downregulated circular RNA hsa_circ_0001649 regulates proliferation, migration and invasion in cholangiocarcinoma cells. Biochem. Biophys. Res. Commun. 2018;496:455–461. doi: 10.1016/j.bbrc.2018.01.077. [DOI] [PubMed] [Google Scholar]
- 7.Yang F., Zhu P., Guo J., Liu X., Wang S., Wang G., Liu W., Wang S., Ge N. Circular RNAs in thoracic diseases. J. Thorac. Dis. 2017;9:5382–5389. doi: 10.21037/jtd.2017.10.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li M., Ding W., Sun T., Tariq M.A., Xu T., Li P., Wang J. Biogenesis of circular RNAs and their roles in cardiovascular development and pathology. FEBS J. 2018;285:220–232. doi: 10.1111/febs.14191. [DOI] [PubMed] [Google Scholar]
- 9.Jeck W.R., Sorrentino J.A., Wang K., Slevin M.K., Burd C.E., Liu J., Marzluff W.F., Sharpless N.E. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA. 2013;19:141–157. doi: 10.1261/rna.035667.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Memczak S., Papavasileiou P., Peters O., Rajewsky N. Identification and Characterization of Circular RNAs As a New Class of Putative Biomarkers in Human Blood. PLoS ONE. 2015;10:e0141214. doi: 10.1371/journal.pone.0141214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ashwal-Fluss R., Meyer M., Pamudurti N.R., Ivanov A., Bartok O., Hanan M., Evantal N., Memczak S., Rajewsky N., Kadener S. circRNA biogenesis competes with pre-mRNA splicing. Mol. Cell. 2014;56:55–66. doi: 10.1016/j.molcel.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 12.Wang X., Zhu X., Zhang H., Wei S., Chen Y., Chen Y., Wang F., Fan X., Han S., Wu G. Increased circular RNA hsa_circ_0012673 acts as a sponge of miR-22 to promote lung adenocarcinoma proliferation. Biochem. Biophys. Res. Commun. 2018;496:1069–1075. doi: 10.1016/j.bbrc.2018.01.126. [DOI] [PubMed] [Google Scholar]
- 13.Du W.W., Yang W., Chen Y., Wu Z.K., Foster F.S., Yang Z., Li X., Yang B.B. Foxo3 circular RNA promotes cardiac senescence by modulating multiple factors associated with stress and senescence responses. Eur. Heart J. 2017;38:1402–1412. doi: 10.1093/eurheartj/ehw001. [DOI] [PubMed] [Google Scholar]
- 14.Yang Y., Fan X., Mao M., Song X., Wu P., Zhang Y., Jin Y., Yang Y., Chen L.L., Wang Y. Extensive translation of circular RNAs driven by N6-methyladenosine. Cell Res. 2017;27:626–641. doi: 10.1038/cr.2017.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Panda A.C. Circular RNAs Act as miRNA Sponges. Adv. Exp. Med. Biol. 2018;1087:67–79. doi: 10.1007/978-981-13-1426-1_6. [DOI] [PubMed] [Google Scholar]
- 16.Hsiao K.Y., Sun H.S., Tsai S.J. Circular RNA - New member of noncoding RNA with novel functions. Exp. Biol. Med. (Maywood) 2017;242:1136–1141. doi: 10.1177/1535370217708978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qu S., Yang X., Li X., Wang J., Gao Y., Shang R., Sun W., Dou K., Li H. Circular RNA: A new star of noncoding RNAs. Cancer Lett. 2015;365:141–148. doi: 10.1016/j.canlet.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 18.Gao J., Xu W., Wang J., Wang K., Li P. The Role and Molecular Mechanism of Non-Coding RNAs in Pathological Cardiac Remodeling. Int. J. Mol. Sci. 2017;18:608. doi: 10.3390/ijms18030608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salmena L., Poliseno L., Tay Y., Kats L., Pandolfi P.P. A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language? Cell. 2011;146:353–358. doi: 10.1016/j.cell.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poliseno L., Salmena L., Zhang J., Carver B., Haveman W.J., Pandolfi P.P. A coding-independent function of gene and pseudogene mRNAs regulates tumour biology. Nature. 2010;465:1033–1038. doi: 10.1038/nature09144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hansen T.B., Jensen T.I., Clausen B.H., Bramsen J.B., Finsen B., Damgaard C.K., Kjems J. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495:384–388. doi: 10.1038/nature11993. [DOI] [PubMed] [Google Scholar]
- 22.Su Q., Lv X. Revealing new landscape of cardiovascular disease through circular RNA-miRNA-mRNA axis. Genomics. 2020;112:1680–1685. doi: 10.1016/j.ygeno.2019.10.006. [DOI] [PubMed] [Google Scholar]
- 23.Bruins M.J., Van Dael P., Eggersdorfer M. The Role of Nutrients in Reducing the Risk for Noncommunicable Diseases during Aging. Nutrients. 2019;11:85–108. doi: 10.3390/nu11010085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou Z., Du D., Chen A., Zhu L. Circular RNA expression profile of articular chondrocytes in an IL-1β-induced mouse model of osteoarthritis. Gene. 2018;644:20–26. doi: 10.1016/j.gene.2017.12.020. [DOI] [PubMed] [Google Scholar]
- 25.Qu S., Liu Z., Yang X., Zhou J., Yu H., Zhang R., Li H. The emerging functions and roles of circular RNAs in cancer. Cancer Lett. 2018;414:301–309. doi: 10.1016/j.canlet.2017.11.022. [DOI] [PubMed] [Google Scholar]
- 26.Ji Q., Zhang C., Sun X., Li Q. Circular RNAs function as competing endogenous RNAs in multiple types of cancer. Oncol. Lett. 2018;15:23–30. doi: 10.3892/ol.2017.7348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou Z.B., Du D., Huang G.X., Chen A., Zhu L. Circular RNA Atp9b, a competing endogenous RNA, regulates the progression of osteoarthritis by targeting miR-138-5p. Gene. 2018;646:203–209. doi: 10.1016/j.gene.2017.12.064. [DOI] [PubMed] [Google Scholar]
- 28.Zheng F., Yu X., Huang J., Dai Y. Circular RNA expression profiles of peripheral blood mononuclear cells in rheumatoid arthritis patients, based on microarray chip technology. Mol. Med. Rep. 2017;16:8029–8036. doi: 10.3892/mmr.2017.7638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao Z., Li X., Jian D., Hao P., Rao L., Li M. Hsa_circ_0054633 in peripheral blood can be used as a diagnostic biomarker of pre-diabetes and type 2 diabetes mellitus. Acta Diabetol. 2017;54:237–245. doi: 10.1007/s00592-016-0943-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Darnell J.E., Jr. Reflections on the history of pre-mRNA processing and highlights of current knowledge: a unified picture. RNA. 2013;19:443–460. doi: 10.1261/rna.038596.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang C.M., Zhang M., Huang L., Hu Z.Q., Zhu J.N., Xiao Z., Zhang Z., Lin Q.X., Zheng X.L., -Yang M. CircRNA_000203 enhances the expression of fibrosis-associated genes by derepressing targets of miR-26b-5p, Col1a2 and CTGF, in cardiac fibroblasts. Sci. Rep. 2017;7:40342–40350. doi: 10.1038/srep40342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xie L., Mao M., Xiong K., Jiang B. Circular RNAs: A Novel Player in Development and Disease of the Central Nervous System. Front. Cell. Neurosci. 2017;11:354–373. doi: 10.3389/fncel.2017.00354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen B., Huang S. Circular RNA: An emerging non-coding RNA as a regulator and biomarker in cancer. Cancer Lett. 2018;418:41–50. doi: 10.1016/j.canlet.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 34.Qian Z., Liu H., Li M., Shi J., Li N., Zhang Y., Zhang X., Lv J., Xie X., Bai Y. Potential Diagnostic Power of Blood Circular RNA Expression in Active Pulmonary Tuberculosis. EBioMedicine. 2018;27:18–26. doi: 10.1016/j.ebiom.2017.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Conn S.J., Pillman K.A., Toubia J., Conn V.M., Salmanidis M., Phillips C.A., Roslan S., Schreiber A.W., Gregory P.A., Goodall G.J. The RNA binding protein quaking regulates formation of circRNAs. Cell. 2015;160:1125–1134. doi: 10.1016/j.cell.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 36.Errichelli L., Dini Modigliani S., Laneve P., Colantoni A., Legnini I., Capauto D., Rosa A., De Santis R., Scarfò R., Peruzzi G. FUS affects circular RNA expression in murine embryonic stem cell-derived motor neurons. Nat. Commun. 2017;8:14741–14749. doi: 10.1038/ncomms14741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khan M.A., Reckman Y.J., Aufiero S., van den Hoogenhof M.M., van der Made I., Beqqali A., Koolbergen D.R., Rasmussen T.B., van der Velden J., Creemers E.E., Pinto Y.M. RBM20 Regulates Circular RNA Production From the Titin Gene. Circ. Res. 2016;119:996–1003. doi: 10.1161/CIRCRESAHA.116.309568. [DOI] [PubMed] [Google Scholar]
- 38.Rybak-Wolf A., Stottmeister C., Glažar P., Jens M., Pino N., Giusti S., Hanan M., Behm M., Bartok O., Ashwal-Fluss R. Circular RNAs in the Mammalian Brain Are Highly Abundant, Conserved, and Dynamically Expressed. Mol. Cell. 2015;58:870–885. doi: 10.1016/j.molcel.2015.03.027. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y., Zhang X.O., Chen T., Xiang J.F., Yin Q.F., Xing Y.H., Zhu S., Yang L., Chen L.L. Circular intronic long noncoding RNAs. Mol. Cell. 2013;51:792–806. doi: 10.1016/j.molcel.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 40.Fan X., Weng X., Zhao Y., Chen W., Gan T., Xu D. Circular RNAs in Cardiovascular Disease: An Overview. BioMed Res. Int. 2017;2017:5135781. doi: 10.1155/2017/5135781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li H., Yang J., Wei X., Song C., Dong D., Huang Y., Lan X., Plath M., Lei C., Ma Y. CircFUT10 reduces proliferation and facilitates differentiation of myoblasts by sponging miR-133a. J. Cell. Physiol. 2018;233:4643–4651. doi: 10.1002/jcp.26230. [DOI] [PubMed] [Google Scholar]
- 42.Lei K., Bai H., Wei Z., Xie C., Wang J., Li J., Chen Q. The mechanism and function of circular RNAs in human diseases. Exp. Cell Res. 2018;368:147–158. doi: 10.1016/j.yexcr.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 43.Li L.J., Leng R.X., Fan Y.G., Pan H.F., Ye D.Q. Translation of noncoding RNAs: Focus on lncRNAs, pri-miRNAs, and circRNAs. Exp. Cell Res. 2017;361:1–8. doi: 10.1016/j.yexcr.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 44.Han D., Li J., Wang H., Su X., Hou J., Gu Y., Qian C., Lin Y., Liu X., Huang M. Circular RNA circMTO1 acts as the sponge of microRNA-9 to suppress hepatocellular carcinoma progression. Hepatology. 2017;66:1151–1164. doi: 10.1002/hep.29270. [DOI] [PubMed] [Google Scholar]
- 45.Huang Z.K., Yao F.Y., Xu J.Q., Deng Z., Su R.G., Peng Y.P., Luo Q., Li J.M. Microarray Expression Profile of Circular RNAs in Peripheral Blood Mononuclear Cells from Active Tuberculosis Patients. Cell. Physiol. Biochem. 2018;45:1230–1240. doi: 10.1159/000487454. [DOI] [PubMed] [Google Scholar]
- 46.Zheng Q., Bao C., Guo W., Li S., Chen J., Chen B., Luo Y., Lyu D., Li Y., Shi G. Circular RNA profiling reveals an abundant circHIPK3 that regulates cell growth by sponging multiple miRNAs. Nat. Commun. 2016;7:11215–11223. doi: 10.1038/ncomms11215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen B., Yu J., Guo L., Byers M.S., Wang Z., Chen X., Xu H., Nie Q. Circular RNA circHIPK3 Promotes the Proliferation and Differentiation of Chicken Myoblast Cells by Sponging miR-30a-3p. Cells. 2019;8:177. doi: 10.3390/cells8020177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li Y., Zheng F., Xiao X., Xie F., Tao D., Huang C., Liu D., Wang M., Wang L., Zeng F., Jiang G. CircHIPK3 sponges miR-558 to suppress heparanase expression in bladder cancer cells. EMBO Rep. 2017;18:1646–1659. doi: 10.15252/embr.201643581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gems D., Partridge L. Genetics of longevity in model organisms: debates and paradigm shifts. Annu. Rev. Physiol. 2013;75:621–644. doi: 10.1146/annurev-physiol-030212-183712. [DOI] [PubMed] [Google Scholar]
- 50.Lipska K.J., Krumholz H., Soones T., Lee S.J. Polypharmacy in the Aging Patient: A Review of Glycemic Control in Older Adults With Type 2 Diabetes. JAMA. 2016;315:1034–1045. doi: 10.1001/jama.2016.0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen J., Zou Q., Lv D., Wei Y., Raza M.A., Chen Y., Li P., Xi X., Xu H., Wen A. Comprehensive transcriptional landscape of porcine cardiac and skeletal muscles reveals differences of aging. Oncotarget. 2017;9:1524–1541. doi: 10.18632/oncotarget.23290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang J.L., Qin M.C., Zhou Y., Xu Z.H., Yang S.M., Zhang F., Zhong J., Liang M.K., Chen B., Zhang W.Y. Comprehensive analysis of differentially expressed profiles of Alzheimer’s disease associated circular RNAs in an Alzheimer’s disease mouse model. Aging (Albany NY) 2018;10:253–265. doi: 10.18632/aging.101387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang H.D., Jiang L.H., Sun D.W., Hou J.C., Ji Z.L. CircRNA: a novel type of biomarker for cancer. Breast Cancer. 2018;25:1–7. doi: 10.1007/s12282-017-0793-9. [DOI] [PubMed] [Google Scholar]
- 54.Patop I.L., Kadener S. circRNAs in Cancer. Curr. Opin. Genet. Dev. 2018;48:121–127. doi: 10.1016/j.gde.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Luo Y.H., Zhu X.Z., Huang K.W., Zhang Q., Fan Y.X., Yan P.W., Wen J. Emerging roles of circular RNA hsa_circ_0000064 in the proliferation and metastasis of lung cancer. Biomed. Pharmacother. 2017;96:892–898. doi: 10.1016/j.biopha.2017.12.015. [DOI] [PubMed] [Google Scholar]
- 56.Chen J., Xu S., Chen S., Zong Z., Han X., Zhao Y., Shang H. CircPUM1 promotes the malignant behavior of lung adenocarcinoma by regulating miR-326. Biochem. Biophys. Res. Commun. 2019;508:844–849. doi: 10.1016/j.bbrc.2018.11.176. [DOI] [PubMed] [Google Scholar]
- 57.Jiang M.M., Mai Z.T., Wan S.Z., Chi Y.M., Zhang X., Sun B.H., Di Q.G. Microarray profiles reveal that circular RNA hsa_circ_0007385 functions as an oncogene in non-small cell lung cancer tumorigenesis. J. Cancer Res. Clin. Oncol. 2018;144:667–674. doi: 10.1007/s00432-017-2576-2. [DOI] [PubMed] [Google Scholar]
- 58.Li W., Jiang W., Liu T., Lv J., Guan J. Enhanced expression of circ_0000735 forecasts clinical severity in NSCLC and promotes cell progression via sponging miR-1179 and miR-1182. Biochem. Biophys. Res. Commun. 2019;510:467–471. doi: 10.1016/j.bbrc.2019.01.134. [DOI] [PubMed] [Google Scholar]
- 59.Tian F., Yu C.T., Ye W.D., Wang Q. Cinnamaldehyde induces cell apoptosis mediated by a novel circular RNA hsa_circ_0043256 in non-small cell lung cancer. Biochem. Biophys. Res. Commun. 2017;493:1260–1266. doi: 10.1016/j.bbrc.2017.09.136. [DOI] [PubMed] [Google Scholar]
- 60.Zhang X., Zhou H., Jing W., Luo P., Qiu S., Liu X., Zhu M., Liang C., Yu M., Tu J. The Circular RNA hsa_circ_0001445 Regulates the Proliferation and Migration of Hepatocellular Carcinoma and May Serve as a Diagnostic Biomarker. Dis. Markers. 2018;2018:3073467. doi: 10.1155/2018/3073467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang X.Y., Huang Z.L., Xu Y.H., Zheng Q., Chen Z., Song W., Zhou J., Tang Z.Y., Huang X.Y. Comprehensive circular RNA profiling reveals the regulatory role of the circRNA-100338/miR-141-3p pathway in hepatitis B-related hepatocellular carcinoma. Sci. Rep. 2017;7:5428–5437. doi: 10.1038/s41598-017-05432-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Deng N., Li L., Gao J., Zhou J., Wang Y., Wang C., Liu Y. Hsa_circ_0009910 promotes carcinogenesis by promoting the expression of miR-449a target IL6R in osteosarcoma. Biochem. Biophys. Res. Commun. 2018;495:189–196. doi: 10.1016/j.bbrc.2017.11.028. [DOI] [PubMed] [Google Scholar]
- 63.Qi H., Sun Y., Jiang Y., Li X. Upregulation of circular RNA circ_0000502 predicts unfavorable prognosis in osteosarcoma and facilitates cell progression via sponging miR-1238. J. Cell. Biochem. 2018;120:8475–8482. doi: 10.1002/jcb.28134. [DOI] [PubMed] [Google Scholar]
- 64.Nie W.B., Zhao L.M., Guo R., Wang M.X., Ye F.G. Circular RNA circ-NT5C2 acts as a potential novel biomarker for prognosis of osteosarcoma. Eur. Rev. Med. Pharmacol. Sci. 2018;22:6239–6244. doi: 10.26355/eurrev_201810_16030. [DOI] [PubMed] [Google Scholar]
- 65.Zhang J., Liu H., Hou L., Wang G., Zhang R., Huang Y., Chen X., Zhu J. Circular RNA_LARP4 inhibits cell proliferation and invasion of gastric cancer by sponging miR-424-5p and regulating LATS1 expression. Mol. Cancer. 2017;16:151–166. doi: 10.1186/s12943-017-0719-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rong D., Lu C., Zhang B., Fu K., Zhao S., Tang W., Cao H. CircPSMC3 suppresses the proliferation and metastasis of gastric cancer by acting as a competitive endogenous RNA through sponging miR-296-5p. Mol. Cancer. 2019;18:25–37. doi: 10.1186/s12943-019-0958-6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 67.Liu H., Liu Y., Bian Z., Zhang J., Zhang R., Chen X., Huang Y., Wang Y., Zhu J. Circular RNA YAP1 inhibits the proliferation and invasion of gastric cancer cells by regulating the miR-367-5p/p27 Kip1 axis. Mol. Cancer. 2018;17:151–165. doi: 10.1186/s12943-018-0902-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sun H., Xi P., Sun Z., Wang Q., Zhu B., Zhou J., Jin H., Zheng W., Tang W., Cao H., Cao X. Circ-SFMBT2 promotes the proliferation of gastric cancer cells through sponging miR-182-5p to enhance CREB1 expression. Cancer Manag. Res. 2018;10:5725–5734. doi: 10.2147/CMAR.S172592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lü L., Sun J., Shi P., Kong W., Xu K., He B., Zhang S., Wang J. Identification of circular RNAs as a promising new class of diagnostic biomarkers for human breast cancer. Oncotarget. 2017;8:44096–44107. doi: 10.18632/oncotarget.17307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang H., Xiao Y., Wu L., Ma D. Comprehensive circular RNA profiling reveals the regulatory role of the circRNA-000911/miR-449a pathway in breast carcinogenesis. Int. J. Oncol. 2018;52:743–754. doi: 10.3892/ijo.2018.4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhao J., Zou H., Han C., Ma J., Zhao J., Tang J. Circlular RNA BARD1 (Hsa_circ_0001098) overexpression in breast cancer cells with TCDD treatment could promote cell apoptosis via miR-3942/BARD1 axis. Cell Cycle. 2018;17:2731–2744. doi: 10.1080/15384101.2018.1556058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu Z., Zhou Y., Liang G., Ling Y., Tan W., Tan L., Andrews R., Zhong W., Zhang X., Song E., Gong C. Circular RNA hsa_circ_001783 regulates breast cancer progression via sponging miR-200c-3p. Cell Death Dis. 2019;10:55–69. doi: 10.1038/s41419-018-1287-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang H.D., Jiang L.H., Hou J.C., Zhong S.L., Zhou S.Y., Zhu L.P., Li J., Wang D.D., Sun D.W., Ji Z.L., Tang J.H. Circular RNA hsa_circ_0052112 promotes cell migration and invasion by acting as sponge for miR-125a-5p in breast cancer. Biomed. Pharmacother. 2018;107:1342–1353. doi: 10.1016/j.biopha.2018.08.030. [DOI] [PubMed] [Google Scholar]
- 74.Xu Y., Yao Y., Leng K., Ji D., Qu L., Liu Y., Cui Y. Increased Expression of Circular RNA circ_0005230 Indicates Dismal Prognosis in Breast Cancer and Regulates Cell Proliferation and Invasion via miR-618/ CBX8 Signal Pathway. Cell. Physiol. Biochem. 2018;51:1710–1722. doi: 10.1159/000495675. [DOI] [PubMed] [Google Scholar]
- 75.Xu J.Z., Shao C.C., Wang X.J., Zhao X., Chen J.Q., Ouyang Y.X., Feng J., Zhang F., Huang W.H., Ying Q. circTADA2As suppress breast cancer progression and metastasis via targeting miR-203a-3p/SOCS3 axis. Cell Death Dis. 2019;10:175–191. doi: 10.1038/s41419-019-1382-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang R., Xing L., Zheng X., Sun Y., Wang X., Chen J. The circRNA circAGFG1 acts as a sponge of miR-195-5p to promote triple-negative breast cancer progression through regulating CCNE1 expression. Mol. Cancer. 2019;18:4–23. doi: 10.1186/s12943-018-0933-7. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 77.Zhong Z., Huang M., Lv M., He Y., Duan C., Zhang L., Chen J. Circular RNA MYLK as a competing endogenous RNA promotes bladder cancer progression through modulating VEGFA/VEGFR2 signaling pathway. Cancer Lett. 2017;403:305–317. doi: 10.1016/j.canlet.2017.06.027. [DOI] [PubMed] [Google Scholar]
- 78.Chen X., Chen R.X., Wei W.S., Li Y.H., Feng Z.H., Tan L., Chen J.W., Yuan G.J., Chen S.L., Guo S.J. PRMT5 Circular RNA Promotes Metastasis of Urothelial Carcinoma of the Bladder through Sponging miR-30c to Induce Epithelial-Mesenchymal Transition. Clin. Cancer Res. 2018;24:6319–6330. doi: 10.1158/1078-0432.CCR-18-1270. [DOI] [PubMed] [Google Scholar]
- 79.Zhang R., Xu J., Zhao J., Wang X. Silencing of hsa_circ_0007534 suppresses proliferation and induces apoptosis in colorectal cancer cells. Eur. Rev. Med. Pharmacol. Sci. 2018;22:118–126. doi: 10.26355/eurrev_201801_14108. [DOI] [PubMed] [Google Scholar]
- 80.Ge Z., Li L.F., Wang C.Y., Wang Y., Ma W.L. CircMTO1 inhibits cell proliferation and invasion by regulating Wnt/β-catenin signaling pathway in colorectal cancer. Eur. Rev. Med. Pharmacol. Sci. 2018;22:8203–8209. doi: 10.26355/eurrev_201812_16513. [DOI] [PubMed] [Google Scholar]
- 81.Chen L., Zhang S., Wu J., Cui J., Zhong L., Zeng L., Ge S. Retraction Note: circRNA_100290 plays a role in oral cancer by functioning as a sponge of the miR-29 family. Oncogene. 2019;38:5750. doi: 10.1038/s41388-019-0828-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zheng J., Liu X., Xue Y., Gong W., Ma J., Xi Z., Que Z., Liu Y. TTBK2 circular RNA promotes glioma malignancy by regulating miR-217/HNF1β/Derlin-1 pathway. J. Hematol. Oncol. 2017;10:52–71. doi: 10.1186/s13045-017-0422-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhao Z., Li X., Gao C., Jian D., Hao P., Rao L., Li M. Peripheral blood circular RNA hsa_circ_0124644 can be used as a diagnostic biomarker of coronary artery disease. Sci. Rep. 2017;7:39918–39926. doi: 10.1038/srep39918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang K., Long B., Liu F., Wang J.X., Liu C.Y., Zhao B., Zhou L.Y., Sun T., Wang M., Yu T. A circular RNA protects the heart from pathological hypertrophy and heart failure by targeting miR-223. Eur. Heart J. 2016;37:2602–2611. doi: 10.1093/eurheartj/ehv713. [DOI] [PubMed] [Google Scholar]
- 85.Salgado-Somoza A., Zhang L., Vausort M., Devaux Y. The circular RNA MICRA for risk stratification after myocardial infarction. Int. J. Cardiol. Heart Vasc. 2017;17:33–36. doi: 10.1016/j.ijcha.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lin F., Zhao G., Chen Z., Wang X., Lv F., Zhang Y., Yang X., Liang W., Cai R., Li J. circRNA-miRNA association for coronary heart disease. Mol. Med. Rep. 2019;19:2527–2536. doi: 10.3892/mmr.2019.9905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li M., Ding W., Tariq M.A., Chang W., Zhang X., Xu W., Hou L., Wang Y., Wang J. A circular transcript of ncx1 gene mediates ischemic myocardial injury by targeting miR-133a-3p. Theranostics. 2018;8:5855–5869. doi: 10.7150/thno.27285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhou B., Yu J.W. A novel identified circular RNA, circRNA_010567, promotes myocardial fibrosis via suppressing miR-141 by targeting TGF-β1. Biochem. Biophys. Res. Commun. 2017;487:769–775. doi: 10.1016/j.bbrc.2017.04.044. [DOI] [PubMed] [Google Scholar]
- 89.Deng Y.Y., Zhang W.P., She J.Q., Zhang L.S., Chen T., Zhou J., Yuan Z.Y. GW27-e1167 Circular RNA Related to PPARγ Function as ceRNA of microRNA in Human Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2016;68(Suppl 16):C51–C52. [Google Scholar]
- 90.Zhou Z.B., Niu Y.L., Huang G.X., Lu J.J., Chen A., Zhu L. Silencing of circRNA.2837 Plays a Protective Role in Sciatic Nerve Injury by Sponging the miR-34 Family via Regulating Neuronal Autophagy. Mol. Ther. Nucleic Acids. 2018;12:718–729. doi: 10.1016/j.omtn.2018.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lin S.P., Ye S., Long Y., Fan Y., Mao H.F., Chen M.T., Ma Q.J. Circular RNA expression alterations are involved in OGD/R-induced neuron injury. Biochem. Biophys. Res. Commun. 2016;471:52–56. doi: 10.1016/j.bbrc.2016.01.183. [DOI] [PubMed] [Google Scholar]
- 92.Lukiw W.J. Circular RNA (circRNA) in Alzheimer’s disease (AD) Front. Genet. 2013;4:307. doi: 10.3389/fgene.2013.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhao Y., Alexandrov P.N., Jaber V., Lukiw W.J. Deficiency in the Ubiquitin Conjugating Enzyme UBE2A in Alzheimer’s Disease (AD) is Linked to Deficits in a Natural Circular miRNA-7 Sponge (circRNA; ciRS-7) Genes (Basel) 2016;7:116–124. doi: 10.3390/genes7120116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cogswell J.P., Ward J., Taylor I.A., Waters M., Shi Y., Cannon B., Kelnar K., Kemppainen J., Brown D., Chen C. Identification of miRNA changes in Alzheimer’s disease brain and CSF yields putative biomarkers and insights into disease pathways. J. Alzheimers Dis. 2008;14:27–41. doi: 10.3233/jad-2008-14103. [DOI] [PubMed] [Google Scholar]
- 95.Loring J.F., Wen X., Lee J.M., Seilhamer J., Somogyi R. A gene expression profile of Alzheimer’s disease. DNA Cell Biol. 2001;20:683–695. doi: 10.1089/10445490152717541. [DOI] [PubMed] [Google Scholar]
- 96.Colangelo V., Schurr J., Ball M.J., Pelaez R.P., Bazan N.G., Lukiw W.J. Gene expression profiling of 12633 genes in Alzheimer hippocampal CA1: transcription and neurotrophic factor down-regulation and up-regulation of apoptotic and pro-inflammatory signaling. J. Neurosci. Res. 2002;70:462–473. doi: 10.1002/jnr.10351. [DOI] [PubMed] [Google Scholar]
- 97.Ginsberg S.D., Alldred M.J., Che S. Gene expression levels assessed by CA1 pyramidal neuron and regional hippocampal dissections in Alzheimer’s disease. Neurobiol. Dis. 2012;45:99–107. doi: 10.1016/j.nbd.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kalia L.V., Lang A.E. Parkinson’s disease. Lancet. 2015;386:896–912. doi: 10.1016/S0140-6736(14)61393-3. [DOI] [PubMed] [Google Scholar]
- 99.Choi D.C., Yoo M., Kabaria S., Junn E. MicroRNA-7 facilitates the degradation of alpha-synuclein and its aggregates by promoting autophagy. Neurosci. Lett. 2018;678:118–123. doi: 10.1016/j.neulet.2018.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Junn E., Lee K.W., Jeong B.S., Chan T.W., Im J.Y., Mouradian M.M. Repression of alpha-synuclein expression and toxicity by microRNA-7. Proc. Natl. Acad. Sci. USA. 2009;106:13052–13057. doi: 10.1073/pnas.0906277106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Choi D.C., Chae Y.J., Kabaria S., Chaudhuri A.D., Jain M.R., Li H., Mouradian M.M., Junn E. MicroRNA-7 protects against 1-methyl-4-phenylpyridinium-induced cell death by targeting RelA. J. Neurosci. 2014;34:12725–12737. doi: 10.1523/JNEUROSCI.0985-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Feng Z., Zhang L., Wang S., Hong Q. Circular RNA circDLGAP4 exerts neuroprotective effects via modulating miR-134-5p/CREB pathway in Parkinson’s disease. Biochem. Biophys. Res. Commun. 2020;522:388–394. doi: 10.1016/j.bbrc.2019.11.102. [DOI] [PubMed] [Google Scholar]
- 103.Liu Q., Zhang X., Hu X., Yuan L., Cheng J., Jiang Y., Ao Y. Emerging Roles of circRNA Related to the Mechanical Stress in Human Cartilage Degradation of Osteoarthritis. Mol. Ther. Nucleic Acids. 2017;7:223–230. doi: 10.1016/j.omtn.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu Q., Zhang X., Hu X., Dai L., Fu X., Zhang J., Ao Y. Circular RNA Related to the Chondrocyte ECM Regulates MMP13 Expression by Functioning as a MiR-136 ‘Sponge’ in Human Cartilage Degradation. Sci. Rep. 2016;6:22572–22583. doi: 10.1038/srep22572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shan K., Liu C., Liu B.H., Chen X., Dong R., Liu X., Zhang Y.Y., Liu B., Zhang S.J., Wang J.J. Circular Noncoding RNA HIPK3 Mediates Retinal Vascular Dysfunction in Diabetes Mellitus. Circulation. 2017;136:1629–1642. doi: 10.1161/CIRCULATIONAHA.117.029004. [DOI] [PubMed] [Google Scholar]
- 106.Wan Q.Q., Wu D., Ye Q.F. The expression profiles of circRNAs in lung tissues from rats with lipopolysaccharide-induced acute respiratory distress syndrome: A microarray study. Biochem. Biophys. Res. Commun. 2017;493:684–689. doi: 10.1016/j.bbrc.2017.08.131. [DOI] [PubMed] [Google Scholar]
- 107.Maass P.G., Glažar P., Memczak S., Dittmar G., Hollfinger I., Schreyer L., Sauer A.V., Toka O., Aiuti A., Luft F.C., Rajewsky N. A map of human circular RNAs in clinically relevant tissues. J. Mol. Med. (Berl.) 2017;95:1179–1189. doi: 10.1007/s00109-017-1582-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tang W., Fu K., Sun H., Rong D., Wang H., Cao H. CircRNA microarray profiling identifies a novel circulating biomarker for detection of gastric cancer. Mol. Cancer. 2018;17:137–142. doi: 10.1186/s12943-018-0888-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sun H., Tang W., Rong D., Jin H., Fu K., Zhang W., Liu Z., Cao H., Cao X. Hsa_circ_0000520, a potential new circular RNA biomarker, is involved in gastric carcinoma. Cancer Biomark. 2018;21:299–306. doi: 10.3233/CBM-170379. [DOI] [PubMed] [Google Scholar]
- 110.Liu M., Liu K.D., Zhang L., Cai J., Yao H.W., Bai Y.K., Zhang Z.T. Circ_0009910 regulates growth and metastasis and is associated with poor prognosis in gastric cancer. Eur. Rev. Med. Pharmacol. Sci. 2018;22:8248–8256. doi: 10.26355/eurrev_201812_16519. [DOI] [PubMed] [Google Scholar]
- 111.Liu X.X., Yang Y.E., Liu X., Zhang M.Y., Li R., Yin Y.H., Qu Y.Q. A two-circular RNA signature as a noninvasive diagnostic biomarker for lung adenocarcinoma. J. Transl. Med. 2019;17:50–62. doi: 10.1186/s12967-019-1800-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pan B., Qin J., Liu X., He B., Wang X., Pan Y., Sun H., Xu T., Xu M., Chen X. Identification of Serum Exosomal hsa-circ-0004771 as a Novel Diagnostic Biomarker of Colorectal Cancer. Front. Genet. 2019;10:1096–1114. doi: 10.3389/fgene.2019.01096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yin W.B., Yan M.G., Fang X., Guo J.J., Xiong W., Zhang R.P. Circulating circular RNA hsa_circ_0001785 acts as a diagnostic biomarker for breast cancer detection. Clin. Chim. Acta. 2018;487:363–368. doi: 10.1016/j.cca.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 114.Wu J., Li J., Liu H., Yin J., Zhang M., Yu Z., Miao H. Circulating plasma circular RNAs as novel diagnostic biomarkers for congenital heart disease in children. J. Clin. Lab. Anal. 2019;33:e22998. doi: 10.1002/jcla.22998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Vausort M., Salgado-Somoza A., Zhang L., Leszek P., Scholz M., Teren A., Burkhardt R., Thiery J., Wagner D.R., Devaux Y. Myocardial Infarction-Associated Circular RNA Predicting Left Ventricular Dysfunction. J. Am. Coll. Cardiol. 2016;68:1247–1248. doi: 10.1016/j.jacc.2016.06.040. [DOI] [PubMed] [Google Scholar]
- 116.Ouyang Q., Wu J., Jiang Z., Zhao J., Wang R., Lou A., Zhu D., Shi G.P., Yang M. Microarray Expression Profile of Circular RNAs in Peripheral Blood Mononuclear Cells from Rheumatoid Arthritis Patients. Cell. Physiol. Biochem. 2017;42:651–659. doi: 10.1159/000477883. [DOI] [PubMed] [Google Scholar]
- 117.Luo Q., Zhang L., Li X., Fu B., Deng Z., Qing C., Su R., Xu J., Guo Y., Huang Z., Li J. Identification of circular RNAs hsa_circ_0044235 in peripheral blood as novel biomarkers for rheumatoid arthritis. Clin. Exp. Immunol. 2018;194:118–124. doi: 10.1111/cei.13181. [DOI] [PMC free article] [PubMed] [Google Scholar]