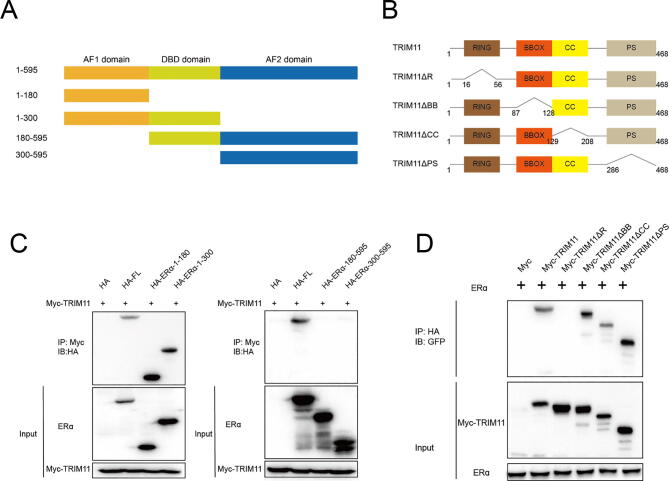
Fig. 4.
TRIM11 associates with ERα AF1 domain through its RING domain. (A, B). ERα and TRIM11 domain structure and deletion mutants used in the study. (C). TRIM11 interacts with ERα through its AF1 domain. HEK293 cells were transfected with 2 µg Myc-TRIM11 together with HA-ER alpha full length or mutants. After 24 h, cells were harvested with NP-40 lysis buffer. Co-IP was performed using Myc antibody. The possible interacted ERα domains were detected by HA antibody. (D). RING domain is required for TRIM11 to interaction with ERα. HEK293 cells were transfected with 2 µg HA-ER alpha together with Myc-TRIM11 full length or mutants. After 24 h, cells were harvested with NP-40 lysis buffer. Co-IP was performed using HA antibody. The possible interacted TRIM11 domains were detected by Myc antibody.