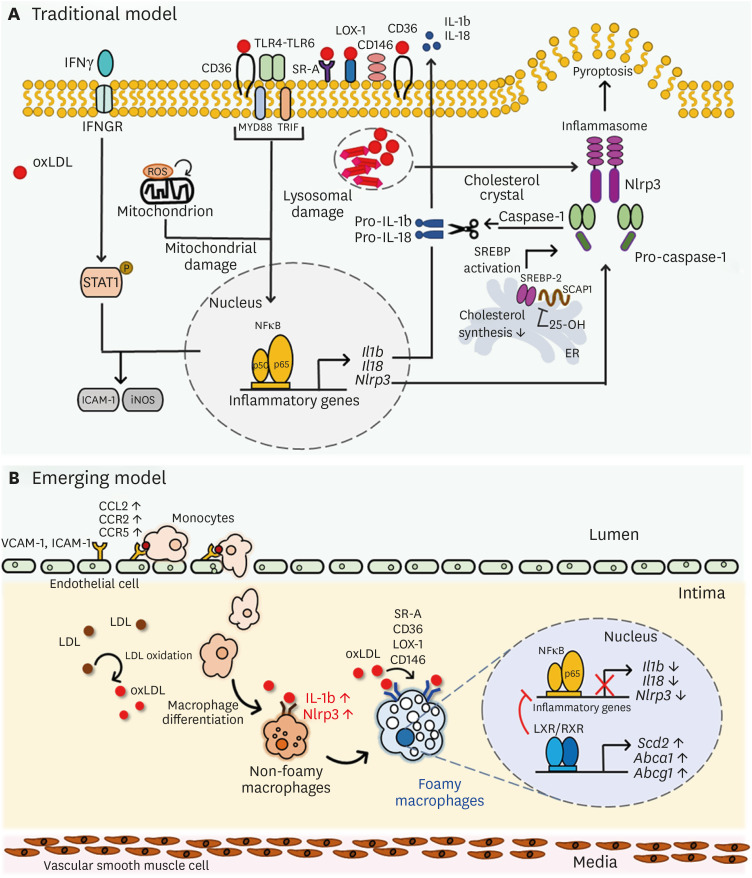
Figure 1. Phenotypic changes of macrophages by lipid uptake in atherosclerotic lesion. (A) Traditional model of oxLDL-mediated inflammatory processes in macrophages. oxLDL activates TLR pathway, leading to NF-κB activation and consecutive pro-inflammatory cytokine secretion such as Il1b, Il18 and Nlrp3. Intracellular ROS accompanied by CD36 signaling leads to mitochondrial metabolic reprogramming and pro-atherogenic signaling, and cholesterol crystals activate NLRP3 and inflammasome formation. (B) An overall diagram describing emerging concept of foam cell formation during hyperlipidemic conditions. Non-foamy macrophages which are newly infiltrated into the intima have low level of cytosolic lipids and show high level of inflammatory genes such as IL-1b and Nlrp3. Foamy macrophages, retained in subintimal space for relatively prolonged time, have high level of lipids in their cytosol and show high level of genes involved in tissue repair and homeostasis, whereas inflammatory genes are relatively downregulated. Uptake of oxidized LDL via scavenger receptors SR-A, CD36, LOX-1, and CD146 induce inflammatory processes in macrophages, leading to production of pro-inflammatory cytokine production. However, after inflammatory responses in early phase, excess cholesterol inhibits Dhcr24, leading to accumulation of desmosterol and LXR/RXR signaling pathway activation. Excess desmosterol also inhibit NF-κB, thereby leading to resolution of acute inflammation. LXR activation promotes resolution of inflammation and cholesterol homeostasis in resolution phages of hyperlipidemic conditions. Unsaturated fatty acids synthesized by LXR pathway inhibit NF-κB -induced pro-inflammation and promote sterol efflux.
LOX-1, lectin-like oxidized LDL rceptor-1; SR, scavenger receptor.