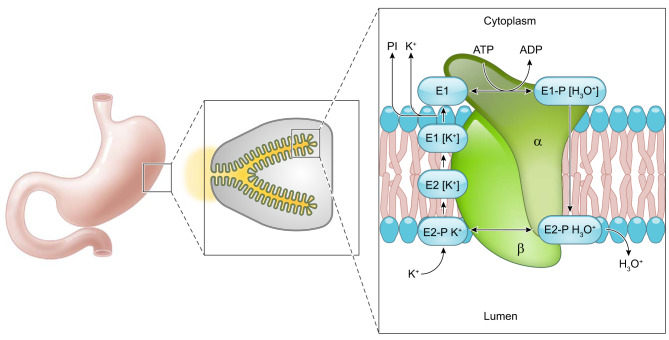
FIGURE 5.
Conformational changes of the H+-K+-ATPase. Phosphorylation and dephosphorylation of the gastric H+-K+-ATPase results in conformational changes facilitating the transport of H3O+ out of parietal cells concurrently with influx of K+. Initially, a hydronium ion binds the cytoplasmic surface of the H+-K+-ATPase and MgATP phosphorylates the protein to form the E1 conformation. In the E1 form, the ion-binding site faces the parietal cell cytoplasm. Next, the E1 form undergoes a conformational change to the E2 form where the ion binding site faces the gastric lumen. In this position the H3O+ is released into the gastric lumen. In this E2 conformation, K+ binds the ion site where H3O+ was previously bound. The enzyme is dephosphorylated, and a conformational change back to the E1 form results in the ion binding site facing the parietal cell cytoplasm where K+ is displaced by ATP binding (386, 387). Based on work on the Na+-K+-ATPase under physiological conditions, the E2 to E1 conformational transition of the unphosphorylated enzyme is postulated to be the rate-limiting step (232).